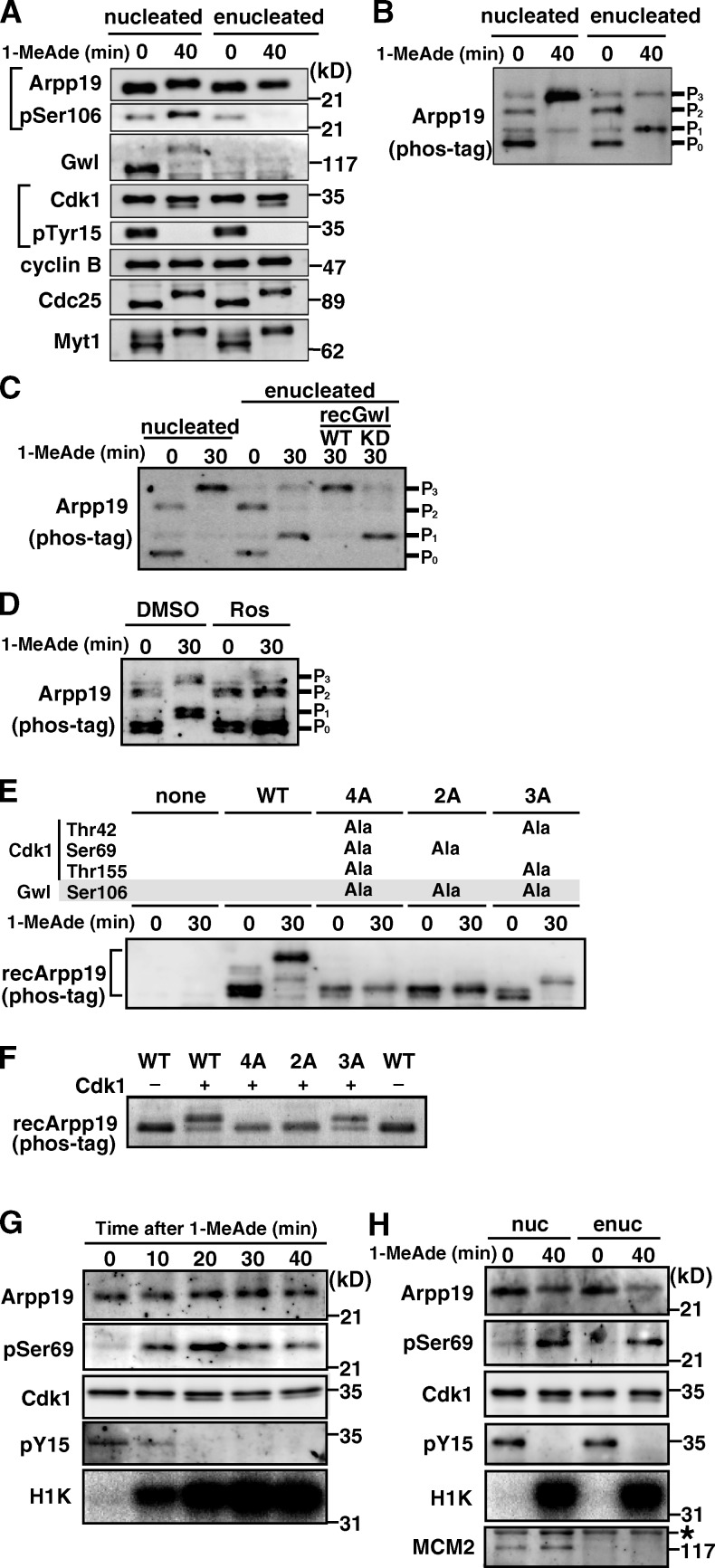
Figure 2.
Arpp19 is phosphorylated on Ser69 by cyclin B–Cdk1 regardless of the presence or absence of Gwl. (A and B) Arpp19 becomes phosphorylated even in enucleated oocytes after 1-MeAde addition. Nucleated or enucleated oocytes were treated with 1-MeAde, and 40 min later (equivalent to metaphase of meiosis I) conventional (A) and phos-tag (B) SDS-PAGE followed by anti-Arpp19 immunoblots were performed. Entry into M phase was confirmed by loss of pTyr15 in Cdk1. (C) Gwl restores enucleation effects on the phosphorylation pattern of Arpp19. Enucleated immature oocytes were uninjected or injected with recombinant Gwl (recGwl; inactive, WT; control kinase-dead, KD) and then treated with 1-MeAde. Phos-tag analysis as in B was performed in C–F. (D) Inhibition of Cdk1 prevents Arpp19 phosphorylation in enucleated oocytes. Enucleated oocytes were treated with 1-MeAde in the presence of the Cdk inhibitor roscovitine (20 µM; Ros) or control DMSO. (E) Arpp19 becomes phosphorylated not only on the Gwl site, Ser106, but also on a possible Cdk1 site, Ser69, after entry into M phase in nucleated oocytes. Nucleated immature oocytes were injected with wild type (WT) or each mutant of Arpp19 proteins (4A [T42A, S69A, S106A, and T155A], 2A [S69A and S106A], and 3A [T42A, S106A, and T155A]), and then treated with 1-MeAde. The 3A mutant, which retains Ser69, showed the band upshift. (F) Cyclin B–Cdk1 directly phosphorylates Arpp19 on Ser69 in vitro. Each of wild-type Arpp19 and the mutant proteins described in E was mixed with purified cyclin B–Cdk1. Wild type and the 3A mutant, both of which retain Ser69, showed the band upshift. (G and H) Arpp19 is phosphorylated on Ser69 in vivo after entry into M phase both in nucleated and enucleated oocytes. Nucleated (G) or enucleated (H) oocytes were treated with 1-MeAde, and oocyte extracts were prepared at the indicated times. Anti-Arpp19 immunoprecipitates were analyzed with anti-pSer69 antibody. Entry into M phase was confirmed by loss of pTyr15 in Cdk1 and by histone H1 kinase (H1K) activation. Enucleation was confirmed by loss of MCM2, a nuclear protein marker (H). Brightness, contrast, and gamma settings were adjusted in the image presentation.