Abstract
MicroRNAs (miRNAs) play important roles in plant growth and development and abiotic stress responses. We report here that heat stress rapidly induces miR398 and reduces transcript of its target gene CSD2. Transgenic plants overexpressing the miR398-resistant form of CSD2 are more sensitive to heat stress than transgenic plants overexpressing normal coding sequence of CSD2. Expression of heat stress transcription factors (HSFs) and heat shock proteins (HSPs) is reduced in the heat-sensitive transgenic plants overexpressing miR398-resistant form of CSD2. Our results suggest that downregulation of CSD2 by the heat-inducible miR398 is required for thermotolerance in Arabidopsis.
Keywords: CSD2, miR398, heat stress-responsive gene expression, thermotolerance, Arabidopsis
Results and Discussion
MicroRNAs (miRNAs) are a class of small non-protein encoding regulatory RNAs ranging from 20 to 24 nucleotides in size that recognize endogenous target mRNAs for degradation or translational repression.1-4 Many plant miRNAs are important for growth and development.5-10 Accumulating evidence showed that miRNAs play essential roles in plant responses to biotic and abiotic stresses.11
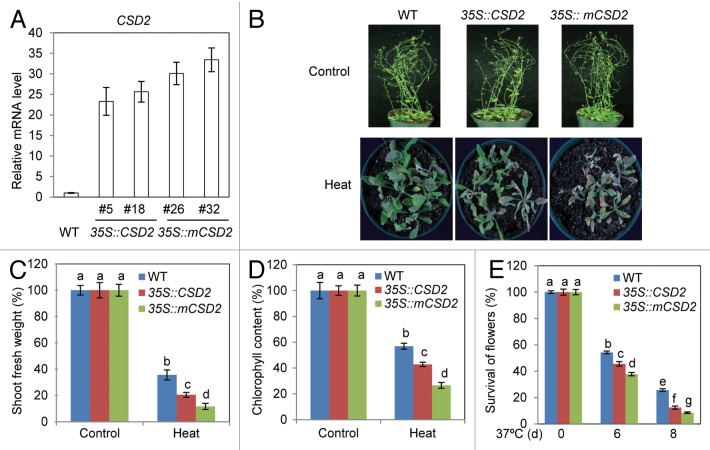
We showed that miR398 is heat-inducible and its target CSD2 is downregulated under heat stress.12 We then generated transgenic Arabidopsis plants overexpressing the miR398-resistant form of CSD2 or normal coding sequence of CSD2 (Fig. 1A). Three-week-old soil-grown transgenic plants overexpressing the miR398-resistant form of CSD2 are more sensitive to heat stress at 37°C compared with wild-type or transgenic plants overexpressing normal coding sequence of CSD2 (Fig. 1B). Relative to wild-type or transgenic plants overexpressing normal coding sequence of CSD2, the transgenic plants overexpressing the miR398-resistant form of CSD2 displayed substantially stunted growth and development in shoot and significantly reduced accumulation of chlorophyll pigments required for photosynthesis (Fig. 1C and D). Plants are especially sensitive to heat stress at reproductive developmental stage. Thus, we examined thermotolerance of flowers of the CSD2 transgenic plants. Flowers of the transgenic plants overexpressing the miR398-resistant form of CSD2 are hypersensitive to heat stress compared with wild-type or transgenic plants overexpressing normal coding sequence of CSD2 (Fig. 1E). These results suggest that heat tolerance requires the downregulation of CSD2.
Figure 1. Thermotolerance of CSD2 transgenic plants. (A) CSD2 expression in transgenic plants expressing normal coding sequence of CSD2 or the miR398-resistant form of CSD2 (mCSD2) under the control of the 35S promoter (these transgenic plants are referred to as CSD2 transgenic plants hereafter). (B) Thermotolerance of wild-type (WT) and CSD2 transgenic plants. Three-week-old soil-grown seedlings were subjected to 0 (control) or 8 d (heat) at 37°C. (C) Shoot fresh weight of WT and CSD2 transgenic plants shown in (B). (D) Chlorophyll content of WT and CSD2 transgenic plants shown in (B). (E) Survival rates of flowers of separate batches of 1-mo-old of WT and CSD2 transgenic plants under heat stress (37°C for 0, 6 or 8 d). Data presented in (B–E) are from one representative individual transgenic line of each transgene. Error bars represent the standard deviation [n = 4 in (A); n = 50–80 in (C–E)]. One-way ANOVA (Tukey-Kramer test) was performed for data in (C–E) and statistically significant differences are indicated by different lowercase letters (p < 0.008).
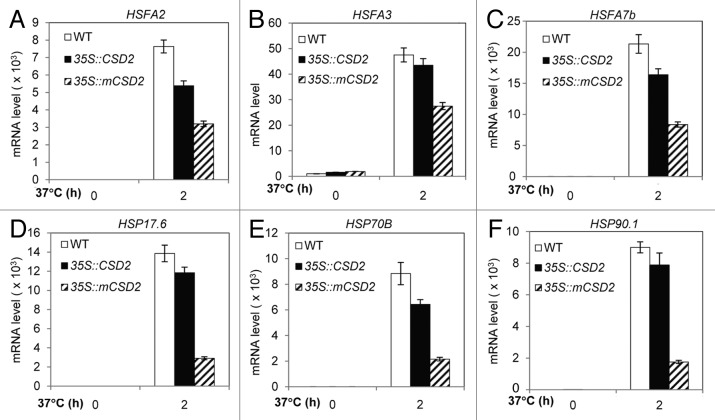
We subsequently analyzed the expression of heat stress-responsive genes in these transgenic plants by qRT-PCR analysis. Compared with their expression in wild-type or transgenic plants overexpressing normal coding sequence of CSD2, expression of HSFA2, HSFA3 and HSFA7b is reduced markedly in transgenic plants overexpressing the miR398-resistant form of CSD2 (Fig. 2A–C). Expression levels of HSP17.6, HSP70B and HSP90.1 are dramatically decreased (relative to their expression in wild-type or transgenic plants overexpressing normal coding sequence of CSD2) in transgenic plants overexpressing the miR398-resistant form of CSD2 (Fig. 2D–F). Effects of CSD2 on expression of HSPs are much stronger in transgenic plants overexpressing the miR398-resistant form of CSD2 (Fig. 2D–F) than transgenic plants expressing the miR398-resistant form of CSD2 under the control of the CSD2 native promoter as described.12 These results indicate that reduced thermotolerance of transgenic plants overexpressing the miR398-resistant form of CSD2 is associated with decreased expression levels of heat stress-responsive genes.
Figure 2. Expression patterns of heat stress-responsive genes in wild-type (WT) and CSD2 transgenic plants. (A–F) Expression of HSFs and HSPs in WT and CSD2 transgenic plants subjected to 0 or 2 h at 37°C. Error bars represent the standard deviation (n = 4). Data in Figure 2 are from one representative individual transgenic line of each transgene. There are two independent transgenic lines per transgene (Fig. 1A).
miR398 also targets CSD1 and CCS (encoding copper chaperone for CSD1 and CSD2) for degradation under heat stress.12 Therefore, we attempted to generate transgenic plants overexpressing the miR398-resistant forms of CSD1 or CCS. However, these two transgenes (CSD1 and CCS) are silenced in the T2 and subsequent generations because of potential unknown posttranscriptional regulation mechanisms.
In summary, our data presented here clearly demonstrate that downregulation of CSD2 by heat-inducible miR398 is required for heat stress-responsive gene expression and thermotolerance in Arabidopsis. Because miR398 family members and their target genes are highly conserved among many eukaryotic plant species,2,12-14 downregulation of CSD2 by heat-inducible miR398 might be a common mechanism by which plants cope with the detrimental effects of heat stress. As a matter of fact, we found that miR398 is induced by heat stress in corn plants.12 Therefore, manipulation of miR398 and/or its target genes might be viable strategies for improving the thermotolerance and yield stability of corn.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant 31060037) to X. Lu and by National Science Foundation (NSF) grants IOS0919745 and MCB0950242 to J. Zhu and by NSF grant DBI0922650.
Glossary
Abbreviations:
- CSD
copper/zinc superoxide dismutase
- CCS
copper chaperone of CSD1 and CSD2
- HSF
heat stress transcription factor
- HSP
heat shock protein
- qRT-PCR
real-time quantitative RT-PCR
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24952
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Beauclair L, Yu A, Bouché N. microRNA-directed cleavage and translational repression of the copper chaperone for superoxide dismutase mRNA in Arabidopsis. Plant J. 2010;62:454–62. doi: 10.1111/j.1365-313X.2010.04162.x. [DOI] [PubMed] [Google Scholar]
- 3.Yu B, Wang H. Translational inhibition by microRNAs in plants. Prog Mol Subcell Biol. 2010;50:41–57. doi: 10.1007/978-3-642-03103-8_3. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Wu G, Poethig RS. Mutations in the GW-repeat protein SUO reveal a developmental function for microRNA-mediated translational repression in Arabidopsis. Proc Natl Acad Sci USA. 2012;109:315–20. doi: 10.1073/pnas.1114673109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones-Rhoades MW, Bartel DP. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell. 2004;14:787–99. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Baker CC, Sieber P, Wellmer F, Meyerowitz EM. The early extra petals1 mutant uncovers a role for microRNA miR164c in regulating petal number in Arabidopsis. Curr Biol. 2005;15:303–15. doi: 10.1016/j.cub.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Lauter N, Kampani A, Carlson S, Goebel M, Moose SP. microRNA172 down-regulates glossy15 to promote vegetative phase change in maize. Proc Natl Acad Sci USA. 2005;102:9412–7. doi: 10.1073/pnas.0503927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- 9.Reyes JL, Chua NH. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007;49:592–606. doi: 10.1111/j.1365-313X.2006.02980.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou GK, Kubo M, Zhong R, Demura T, Ye ZH. Overexpression of miR165 affects apical meristem formation, organ polarity establishment and vascular development in Arabidopsis. Plant Cell Physiol. 2007;48:391–404. doi: 10.1093/pcp/pcm008. [DOI] [PubMed] [Google Scholar]
- 11.Khraiwesh B, Zhu J-K, Zhu J. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta. 2012;1819:137–48. doi: 10.1016/j.bbagrm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Q, Lu X, Zeng H, Zhang Y, Zhu J. Heat stress induction of miR398 triggers a regulatory loop that is critical for thermotolerance in Arabidopsis. Plant J. 2013;74:840–51. doi: 10.1111/tpj.12169. [DOI] [PubMed] [Google Scholar]
- 13.Sunkar R, Zhu J-K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell. 2004;16:2001–19. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dugas DV, Bartel B. Sucrose induction of Arabidopsis miR398 represses two Cu/Zn superoxide dismutases. Plant Mol Biol. 2008;67:403–17. doi: 10.1007/s11103-008-9329-1. [DOI] [PubMed] [Google Scholar]