Figure 4. Negative cooperation effect of NADH binding within the cHAD dimer.
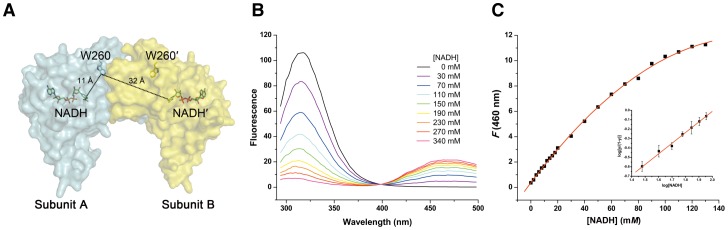
(A) A combined surface and stick model presents the distances between the side chain of intrinsic W260 and bound NADHs of subunit A (cyan) and subunit B (yellow). The positions of NADHs are determined by superposition of the crystal structure of human HAD·NADH complex (PDB entry 1F17) with the present cHAD crystal structure. (B) Fluorescence resonance energy transfer (FRET) spectrum by titrating NADH with the wild type cHAD. The excitation wavelength is 270 nm and the emission wavelength is scanned from 290 to 500 nm. The FRET signal appears at the wavelength of 460 nm. (C) Hyperbolic curve of the FRET fluorescence signal at 460 nm against the titration of NADH concentration for the wild type cHAD. The insert represents the logarithmic Hill plot between 10 and 90% active site saturation.
