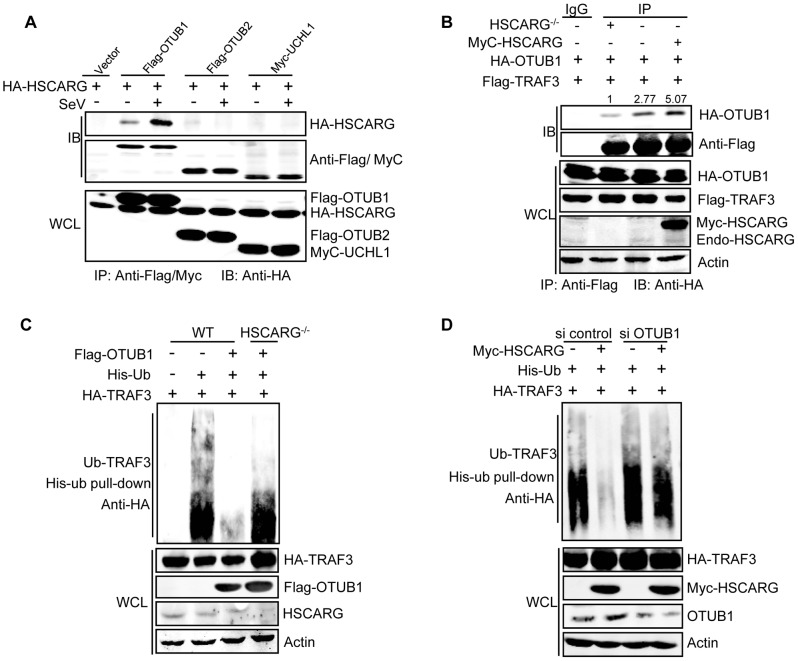
Figure 3. HSCARG and OTUB1 cooperatively inhibit TRAF3 ubiquitination.
(A) HSCARG interacts with OTUB1. HEK293T cells transfected with indicated plasmids were infected with or without SeV (40 HAU/ml) for 6 h. Co-IP analysis was performed with anti-Flag or anti-Myc antibody followed by IB with anti-HA antibody. (B) HSCARG promotes TRAF3-OTUB1 interaction. HSCARG −/− and wild-type HEK293T cells were transfected with Flag-TRAF3, HA-OTUB1, or together with Myc-HSCARG. Co-IP was then performed with anti-Flag and IB with anti-HA to examine the effect of HSCARG on the TRAF3-OTUB1 complex. Quantification of HA-OTUB1 was performed using Odyssey Infrared Imaging System and software Odyssey V3.0. The data was normalized to the enriched protein. (C) OTUB1 predominantly loses its de-ubiquitination ability in HSCARG −/− cells. The wild-type and HSCARG −/− HEK293T cells were transfected with HA-TRAF3, His-ubiquitin, together with or without Flag-OTUB1, and then subjected to His-ubiquitin pull-down analysis and IB with anti-HA to measure TRAF3 ubiquitination level. (D) HSCARG relies on OTUB1 to inhibit TRAF3 ubiquitination. HEK293T cells were transfected with OTUB1 siRNA (40 nM), negative control, and HA-TRAF3, His-ubiquitin with or without Myc-HSCARG as indicated. 72 h later, His-ubiquitin pull-down was performed followed by IB with anti-HA to detect TRAF3 ubiquitination level.