Figure 1.
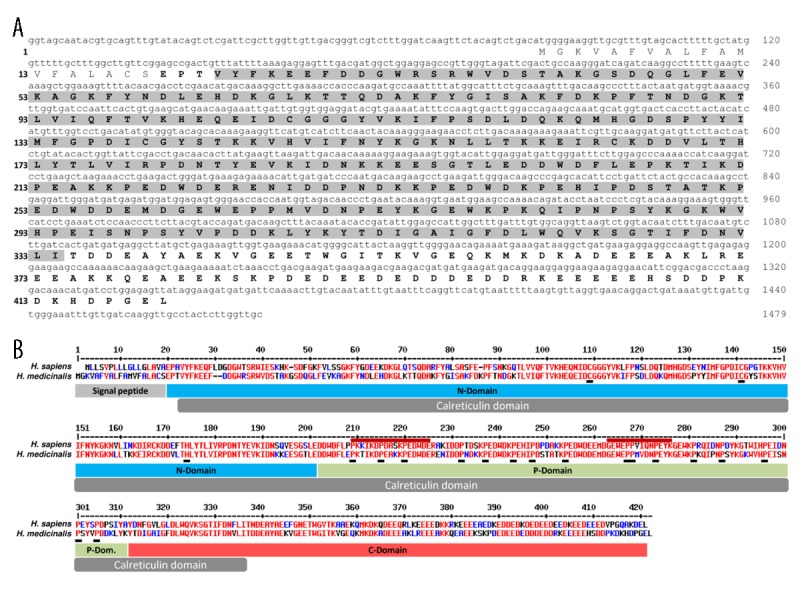
Characterization of a calreticulin-related molecule (HmCalR) in the medicinal leech. (A) Nucleotide and amino acid sequences of leech HmCalR. Numbers of nucleotides and amino acids are indicated on right and left of the sequence, respectively. The protein sequence contains a putative signal peptide (1–19) and a mature form (20–420) containing a Calreticulin-conserved domain (23–334) highlighted in light grey. (B) Sequence alignment with human calreticulin. High and low consensus homologies are represented by red and blue residues, respectively. Signal peptide and calreticulin domain are indicated by grey boxes. The 3 significant domains from human form (N-, P- and C-domains) are indicated by blue, green, and red boxes, respectively. Critical residues (Cysteines and Histidine in N-domain, and Proline in P-domain) are indicated by black boxes. Two amino-acid sequences, named repeats A (PxxIxDPDAxKPEDWDE) and B (GxWxPPxIxNPxYx) in human calreticulin are specified by the upper red lines.
