Abstract
Restless Legs Syndrome (RLS) is a sensory-motor neurological disorder that appears to be surprisingly common in the community. Periodic limb movements in sleep are typically encountered in more than 80% of RLS patients and comprise involuntary muscular jerks in the lower limbs, such as flexion of the knees or ankles. Iron deficiency and dopaminergic neuronal dysfunction in the central nervous system are currently thought to be the likely pathophysiological culprits. There is evidence linking RLS to hypertension and cardiovascular disease. This short review will first present a synopsis of epidemiological, clinical, and pathophysiological data concerning the syndrome, and then information on the possible links between RLS and cardiovascular disorders
MeSH Keywords: Cardiovascular Diseases, Restless Legs Syndrome, Hypertension, Dopamine, Stroke, Coronary Artery Disease
Background
Restless Legs Syndrome (RLS), a term introduced by Karl-Axel Ekbom, is a sensory-motor neurological disorder that appears to be surprisingly common in the community, although patient and even physician awareness of it is generally low. The causes of this syndrome are not clear. Iron deficiency and dopaminergic neuronal dysfunction in the central nervous system are currently thought to be the likely pathophysiological culprits [1]. A series of research studies have provided evidence that possibly links RLS to hypertension and cardiovascular disease (CVD), raising many important questions. This short review will first present a synopsis of epidemiological, clinical, and pathophysiological data concerning the syndrome, and then information on the possible links between RLS and cardiovascular disorders.
Epidemiology of RLS
Large community studies in Europe and North America show RLS prevalence rates from 4% to 29% in the general adult population [2]. Prevalence increases with age and in the presence of coexisting morbidities, and it is higher in women [1]. Research on Asian populations reveals significantly lower prevalence, with reports of 12% in Korea and 2% in India [3,4]. Racial differences in prevalence are not clearly understood and might represent a problem of limited access to special care units in developing countries and a lack of special sleep medicine clinics in many parts of such countries, or may be due to cultural differences in interpreting symptoms. RLS can be categorized as primary or idiopathic and secondary or symptomatic. Primary RLS typically manifests before the age of 45 years and has a strong genetic component. This is suggested by a significantly higher incidence of a positive family history of affected relatives in comparison to secondary RLS, which typically occurs after the age of 45 years and is associated with, or sometimes caused by, other coexisting disorders (coexisting disorders reported in the literature include pregnancy, iron deficiency, peripheral polyneuropathy, obesity, diabetes mellitus, multiple sclerosis, rheumatoid arthritis, Parkinson’s disease, end-stage renal disease, fibromyalgia, chronic obstructive pulmonary disease, obstructive sleep apnea, atopic dermatitis, migraine, chronic liver disease, and depression). The mode of inheritance is not completely known and the genetics of RLS appears complex and heterogenic. It might be the case that multiple genetic loci confer differing contributions to RLS. Various genes, among them MEIS1 and NOS1, are currently under investigation.
Clinical Features
RLS is mainly characterized by a sometimes inescapable urge to move the legs, mainly to find relief from paraesthesias felt deep in the lower legs during periods of relaxation. Patients may complain of unpleasant, creeping or burning sensations, mostly between the knees and ankles, and commonly bilateral and symmetrical, which drive them to move the legs or walk around. The arms and (rarely) other body areas can also be involved. Paraesthesias may last for hours and are especially bothersome late in the evening or early at night, when they can have a deleterious effect on sleep.
Loss of sleep is a serious and unfortunate outcome with multiple consequences, drastically affecting quality of life. Periodic Limb Movements in Sleep (PLMS) are typically encountered in more than 80% of RLS patients and comprise involuntary muscular jerks in the lower limbs, such as flexion of the knees or ankles. The clinical course of RLS is chronic and symptoms tend to increase or worsen.
Restless Legs Syndrome is a diagnosis based on patient history. Physical examination is generally normal but signs or symptoms of causative comorbidities in secondary RLS may be found. The international Restless Legs Syndrome research group (www.irlssg.org) has issued specific diagnostic criteria to facilitate diagnosis (Table 1).
Table 1.
RLS diagnostic criteria (2011 revision, taken from www.irlssg.org).
| RLS essential diagnostic criteria |
|---|
| An urge to move the legs usually but not always accompanied by or felt to be caused by uncomfortable and unpleasant sensations in the legs.1,2 |
| The urge to move the legs and any accompanying unpleasant sensations begin or worsen during periods of rest or inactivity, such as lying down or sitting. |
| The urge to move the legs and any accompanying unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues.3 |
| The urge to move the legs and any accompanying unpleasant sensations during rest or inactivity only occur or are worse in the evening or night than during the day.4 |
| The above features are not solely accounted for as symptoms primary to another medical or a behavioral condition (e.g. myalgia, venous stasis, leg edema, arthritis, leg cramps, positional discomfort, habitual foot tapping).5 |
Sometimes the urge to move the legs is present without the uncomfortable sensations and sometimes the arms or other parts of the body are involved in addition to the legs.
For children, the description of these symptoms should be in the child’s own words.
When symptoms are very severe, relief by activity may not be noticeable but must have been previously present.
When symptoms are very severe, the worsening in the evening or night may not be noticeable but must have been previously present.
These conditions, often referred to as RLS mimics, have been commonly confused with RLS particularly in surveys, because the produce symptoms that meet or at least come very close to meeting all of the above criteria. The list gives some examples of this that have been noted as particularly significant in epidemiological studies and clinical practice. RLS may also occur with any of these conditions, but the RLS symptoms will then be more in degree, conditions of expression or character than those usually occurring as part of the other condition.
Pathophysiology
The pathophysiology of RLS is not fully understood and at present only symptomatic treatment exists.
Three factors are thought to interact: a genetic component, dopaminergic dysfunction in subcortical brain systems, and iron deficiency in the central nervous system.
Dopaminergic dysfunction
The impressive improvement seen in the symptoms of RLS patients who are administered levodopa or centrally acting dopaminergic agonists, together with the exacerbation of symptoms that is seen after administration of dopamine antagonists, indicate that dopaminergic neuronal dysfunction at a subcortical level in the brain may be a key cause of RLS. Thus, research has focused on unraveling potential disturbances of dopamine pathways in RLS. Evidence suggests that the A11 dopaminergic system may be important in the development of the syndrome [5]. The A11 system connects to the suprachiasmatic nucleus of the hypothalamus, involved in the regulation of circadian rhythms, and A11 neurons project into the dorsal horns and intermediolateral tracts of the spinal cord, and are thought to play a role in sensory suppression. A11 dopaminergic dysfunction might thus be involved in the generation of sensory symptoms of RLS.
Dopaminergic dysfunction in RLS is also supported by imaging studies in RLS patients. Positron emission tomography (PET) studies have revealed decreased dopamine D2 receptor binding in the striatum and extrastriatal areas [6,7], although other studies have yielded contradicting results [8,9].
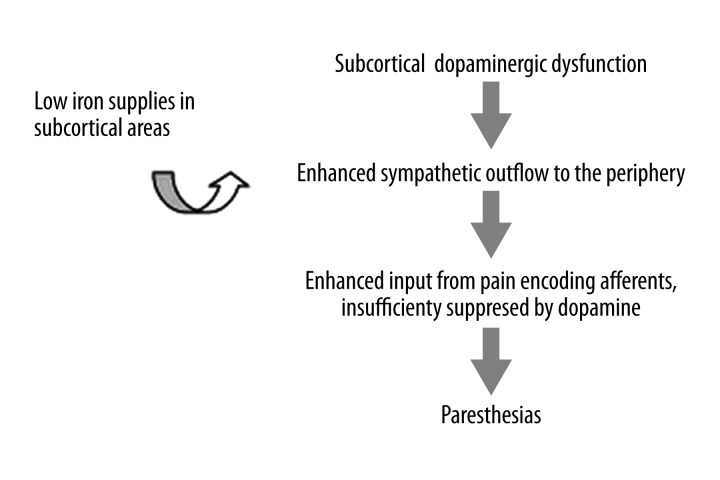
A pathophysiological model of dopaminergic dysfunction in RLS has been hypothesized [10]: Dopamine released by A11 neurons in the spinal cord directly inhibits sympathetic preganglionics in the intermediolateral column and thus a hypofunction of the A11 system will shift the balance towards excitation of the sympathetic neurons by descending serotoninergic cells. The ensuing enhanced sympathetic outflow to the periphery, including somatic muscle fibers, will alter the activity in afferents of the muscle fibers back to the spinal cord. High threshold muscle afferents in lamina I, which are the enhanced input from pain encoding, are insufficiently suppressed in the absence of dopamine or D2-like receptors, resulting in paraesthesias perceived at the cortical level (Figure 1).
Figure 1.
Proposed pathophysiological model for RLS.
Iron deficiency
Iron is a necessary cofactor for the synthesis of dopamine from tyrosine hydroxylase in the brain, and the regulation of dopamine receptors. Many conditions that lead to secondary RLS, such as pregnancy and renal failure, are associated with low iron levels. Some studies have found a significant association between iron deficiency and RLS, but others have shown that RLS can be present while ferritin levels are normal or high. More importantly, some studies have found decreased iron and ferritin levels in the cerebrospinal fluid of RLS patients [11,12], further supporting the role of defective iron metabolic usage in the brain. Magnetic resonance imaging (MRI) studies have also shown reduced iron concentrations in the putamen and substantia nigra areas [13]. Neuropathological examinations have confirmed such results and have shown increased transferrin staining in neuromelanin-containing cells [14]. It has been suggested that the lower the brain iron load, the greater the severity of RLS is, because evidence shows an inverse correlation of RLS severity with the echogenicity of the substantia nigra area, with the echogenicity reflecting nigral iron supplies [15]. Such findings suggest a possible dysregulation of iron intake in brain dopaminergic cells, which would hamper dopamine synthesis and/or the regulation of dopamine transporters and receptors, leading to pathophysiological sequelae.
RLS and Hypertension
Research has raised the possibility of a link between RLS and hypertension, but conflicting data exist; hence, a focus on this subject is warranted. Investigations on RLS and PLMS, and current theoretical models of RLS pathophysiology, implicate sympathetic hyperactivity, a pathophysiological state that has long been studied in the context of hypertension and cardiovascular risk. Sympathetic activity might explain an association of RLS to hypertension and CVD, and a possible effect of RLS on blood pressure and CVD may not simply be the outcome of loss of sleep and the resulting shorter duration of sleep, which is by itself a plausible and researched explanation (Table 2).
Table 2.
Sleep disorders that have been associated with hypertension. For a review, see: Calhoun DA, Harding SM. Sleep and hypertension. Chest, 2010; 138: 434–43.
| Sleep disorders associated with hypertension |
|---|
|
Pioneering work by Ekbom postulated a common vasoconstrictive background for RLS symptoms and often co-occurring cold feet in RLS patients. Later, it was hypothesized that PLMS, once again a common sign in RLS, resulted from sympathetic activation. Hypertension has been reported as frequent among patients with PLMS and the presence of PLMS has been associated with increasing severity of hypertension, even after adjustment for age, sex, obesity, and other factors [16]. This finding has been reconfirmed in a larger study [17], where it was also found that the odds ratio (OR) for hypertension in patients with more than 30 PLMS per hour was 2.26 [95% confidence intervals (CI) 1.28–3.99], similar to the OR of patients with sleep-disordered breathing. Recently, significant periodic rises of blood pressure and heart rate associated with PLMS, even in the absence of cortical arousal, have been reported, and the authors have suggested that PLMS may cause sympathetic autonomic activation and possibly increase the risk for CVD [18,19].
A possible, albeit far from causative, link has thus been established. Epidemiology studies that focused on RLS prevalence in the general population also reported on the prevalence of hypertension in RLS patients. Research in Sweden revealed a greater likelihood of hypertension in male RLS patients compared to controls, with an OR of 1.5 (95% CI 0.9–2.4) [20], and a study enlisting from multiple European countries found a similar OR of 1.36 (95% CI 1.14–1.61) [21]. Moreover, a study in the USA also reported a link between hypertensive patients and RLS [22]. Women with RLS were shown to have a higher prevalence of hypertension, independent of age, body mass index, smoking status, and presence of stroke or myocardial infarction [23–25]. Furthermore, the prevalence was shown to increase in relation to the frequency of symptoms. Limitations in such studies do exist, for example the criteria for RLS diagnosis were not always the same or up-to-date between studies, patients were not interviewed or examined, sometimes RLS-mimicking states were also included, and confounding factors such as obstructive sleep apnea were not always possible to exclude. In addition, other studies have failed to find a correlation between RLS and hypertension [26,27], subjecting this matter to further debate. Population variations, demographics, and the often different criteria and questions used to confirm RLS or hypertension among studies possibly provide some explanation of why conflicting evidence exists. From a pathophysiological standpoint, PLMS seen in RLS patients may be the key in the possible association with hypertension. PLMS has been temporally associated with a rise in pulse rate and blood pressure, with or without cortical arousal seen in electroencephalography (EEG), pinpointing a sympathetic activation [18,19]. Of note, contrary to other hypotheses, one study reported that sympathetic activity is the physiologic process that is functionally and most probably causally related to PLMS. Autonomic activation was shown to start several seconds before movement onset and the authors of this study argue that the increased sympathetic activation, surpassing a certain threshold, facilitates PLMS and may also be involved in arousal control, rather than the PLMS being responsible for an increased sympathetic activation [28]. It is reasonable to postulate that the repeated overnight changes in blood pressure are sympathetically driven and, in part by increasing daytime blood pressure, this increased nocturnal sympathetic activity might be related to hypertension and CVD.
RLS and CVD
Epidemiological studies have also associated RLS with heart disease and stroke [20,21,26,27], but data are not always consistent. The OR for the association of heart disease to RLS has been measured at 2.5 (95% CI 1.4–4.3) [20] and 1.41 (95% CI 1.06–1.88) [21]. The adjusted OR for CVD (coronary artery disease, stroke, and heart failure) for RLS patients was estimated at 2.07 (95% CI 1.43–3.00) after analysis of data from the Sleep Heart Health Study [27]. Analysis for coronary artery disease alone found an adjusted OR of 2.05 (95% CI 1.38–3.04). The authors reported that the association appears stronger in patients with greater frequency or severity of RLS symptoms. Their analysis for CVD factors also showed it was unlikely that the observed association of RLS with CVD was due to confounding by known major CVD risk factors. PLMS have been associated with congestive heart failure in a controlled study [29], but the nature of this association is not completely understood. Regarding stroke, limited research has shown that stroke may be the direct cause of RLS [30,31], and the reverse relationship has also been contemplated, but has not been clearly established [32]. However, in light of the contradicting evidence provided by some studies, with regard to RLS and hypertension, it has been noted that an association between RLS and CVD in general seems more consistent than the association of RLS to hypertension alone. Very recently, a prospective study confirmed already existing cross-sectional data regarding the association of RLS and coronary heart disease, at least in Western populations [33]. The authors note that the duration of the symptoms of RLS is an important factor for the association, with an elevated risk shown for female Caucasian patients whose symptoms last for at least 3 years. The multivariable-adjusted hazard ratios of women with RLS for ≥3 years were 1.80 (95% CI 1.07–3.01) for non-fatal myocardial infarction and 1.49 (95% CI 0.55–4.04) for fatal coronary heart disease, relative to women without RLS. Another study has found a significant increase of cardiovascular risk factors among RLS patients compared to normal controls [34], which may indicate a reason for increased cardiovascular risk. Female sex (OR 2.16, 95% CI 1.11–4.17), smoking (OR 1.82, 95% CI, 1.10–3.00), and HDL/LDL cholesterol (OR 0.18, 95% CI 0.034–0.90) were significantly associated with RLS compared with subjects without RLS. However, at least as far as dyslipidemia is concerned, there are reports against an altered lipid metabolism as a risk factor for the development of cerebrovascular disease in patients with RLS, associating dyslipidemia with comorbid obstructive sleep apnea (OSA), a condition known to frequently coexist with RLS [35]. The authors point out that previous epidemiological and association studies between RLS and hypertension or CVD may not have taken into account interactions between comorbidities associated with RLS, indicating that this might reflect some of the results linking RLS to these conditions. Moreover, another study found no difference in the prevalence of CVD or CVD risk factors between RLS patients and normal controls [32], and in 2 large prospective studies of health professionals, RLS was not associated with an increased risk of any incident CVD event [36]. The prospective design confers strength in these last 2 studies, but the authors also emphasized that no information on RLS symptom frequency or severity was available, and that only Caucasian professionals were included.
Because hypertension is an established risk factor for heart failure, coronary artery disease, and stroke, an increase of cardiovascular risk caused by RLS-induced hypertension is a hypothesis that may deserve future research. Regarding possible mechanisms of RLS-associated increases in stroke and heart disease risks without hypertension as the intermediary, it has been suggested that the increase of nocturnal blood pressure associated with PLMS, without an overall change in daytime blood pressure, may nevertheless increase the 24-hour blood pressure profile and may also increase coagulability and the risk of atherosclerotic plaque formation and rupture, providing a possible explanation [27]. After all, blood pressure variability and the time rate of 24-hour blood pressure variations have been shown to correlate with atherosclerosis [37]. Also, PLMS frequency, with and without arousal, has been associated with increased incidence of all-cause CVD [38], although this study did not acquire data regarding RLS and could not extrapolate results for RLS patients.
In summary, complex interactions between RLS/PLMS and CVD seem to exist, but one- or even two-way causality has yet to be shown, as most studies that have been conducted were cross-sectional. It may be that duration and severity of RLS are both important in understanding possible relations to CVD. It has not escaped the attention of researchers that CVD might also lead to RLS, by vascular alterations in the central nervous system or the periphery and sympathetic activation. Both conditions share mutual risk factors, such as obesity, chronic stress, smoking, and sleep impairment. It is not unreasonable to suggest screening for RLS in CVD patients, or to consider lifestyle modifications that reduce CVD risk in RLS patients. Much work has been published contemplating the links between RLS and hypertension or CVD, an exhaustive coverage of which is beyond the scope of this short review but can be found elsewhere [39].
Conclusions
RLS is a common disorder in the general population and efforts must be made to improve physician awareness. Because it can have a devastating effect on the quality of life, it is imperative that patients be diagnosed and offered treatment. Although there is no cure, treatment based on administration of dopaminergic drugs is effective and provides relief from symptoms. There is currently limited knowledge concerning the pathogenesis and pathophysiology of primary RLS, but research is ongoing. A considerable amount of data points to an association between RLS, hypertension, and CVD. Some evidence suggests that RLS patients might be at greater risk for cardiovascular events. However, most data come from cross-sectional studies and causality is far from proven. Conflicting evidence exists, confusing the interpretation of results. Future research is expected to shed more light on this potentially very important matter.
Footnotes
Source of support: Departmental sources
Conflicts of interest
The authors have no conflicts of interest to declare. No funding was received for the preparation of the manuscript.
References
- 1.Ekbom K, Ulfberg J. Restless legs syndrome. J Intern Med. 2009;266:419–31. doi: 10.1111/j.1365-2796.2009.02159.x. [DOI] [PubMed] [Google Scholar]
- 2.Innes KE, Selfe TK, Agarwal P. Prevalence of Restless Legs Syndrome in North American and Western European Populations: A Systematic Review. Sleep Med. 2011;12(7):623–34. doi: 10.1016/j.sleep.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Choi C, Shin K, et al. Prevalence of restless legs syndrome and associated factors in the Korean adult population: The Korean Health and Genome Study. Psychiatry Clin Neurosci. 2005;59:350–53. doi: 10.1111/j.1440-1819.2005.01381.x. [DOI] [PubMed] [Google Scholar]
- 4.Rangarajan S, Rangarajan S, D’Souza GA. Restless legs syndrome in an Indian urban population. Sleep Med. 2007;9:88–93. doi: 10.1016/j.sleep.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Trenkwalder C, Paulus W. Why do restless legs occur at rest? Pathophysiology of neuronal structures in RLS. Neurophysiology of RLS (part 2) Clin Neurophysiol. 2004;115:1975–88. doi: 10.1016/j.clinph.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Ruottinen HM, Partinen M, Hublin C, et al. An FDOPA PET study in patients with periodic limb movement disorder and restless legs syndrome. Neurology. 2000;54:502–4. doi: 10.1212/wnl.54.2.502. [DOI] [PubMed] [Google Scholar]
- 7.Cervenka S, Pålhagen SE, Comley RA, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain. 2006;129:2017–28. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- 8.Michaud M, Soucy JP, Chabli A, et al. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. 2002;249:164–70. doi: 10.1007/pl00007859. [DOI] [PubMed] [Google Scholar]
- 9.Trenkwalder C, Walters AS, Hening WA, et al. Positron emission tomographic studies in restless legs syndrome. Mov Disord. 1999;14:141–45. doi: 10.1002/1531-8257(199901)14:1<141::aid-mds1024>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–30. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- 11.Earley CJ, Connor JR, Beard JL, et al. Abnormalities in CSF concentrations of ferritin and transferring in restless legs syndrome. Neurology. 2000;54:1698–700. doi: 10.1212/wnl.54.8.1698. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno S, Mihara T, Miyaoka T, et al. CSF iron, ferritin and transferin levels in restless legs syndrome. J Sleep Res. 2005;14:43–47. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 13.Allen RP, Barker PB, Wehrl F, et al. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56:263–65. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- 14.Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–9. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 15.Pedroso JL, Bor-Seng-Shu E, Felicio AC, et al. Severity of restless legs syndrome is inversely correlated with echogenicity of the substancia nigra in different neurodegenerative movement disorders. A preliminary observation. J Neurol Sci. 2012;319:59–62. doi: 10.1016/j.jns.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Espinar-Sierra J, Vela-Bueno A, Luque-Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry Clin Neurosci. 1997;51:103–7. doi: 10.1111/j.1440-1819.1997.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 17.Billars L, Hicks A, Bliwise D. Hypertension risk and PLMS in restless legs syndrome. Sleep. 2007;30:A297–98. [Google Scholar]
- 18.Siddiqui F, Strus J, Ming X, et al. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Pennestri MH, Montplaisir J, Colombo R, et al. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–18. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 20.Ulfberg J, Nystrom B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001;16:1159–63. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 21.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–54. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 22.Phillips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome. Chest. 2005;129:76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 23.Batool-Anwar S, Malhotra A, Forman J, et al. Restless Legs Syndrome and Hypertension in Middle-Aged Women. Hypertension. 2011;58:791–96. doi: 10.1161/HYPERTENSIONAHA.111.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chhabra A, Aronow WS, Ahn C, et al. Incidence of new cardiovascular events in patients with and without peripheral arterial disease seen in a vascular surgery clinic. Med Sci Monit. 2012;18(3):CR131–34. doi: 10.12659/MSM.882517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrdoljak A, Bergman Marković B, et al. How well do anthropometric indices correlate with cardiovascular risk factors? A cross-sectional study in Croati. Med Sci Monit. 2012;18(2):PH6–11. doi: 10.12659/MSM.882451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7:545–52. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70:35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 28.Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep. 2007;30:755–66. doi: 10.1093/sleep/30.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanly PJ, Zuberi-Khokhar N. Periodic limb movements during sleep in patients with congestive heart failure. Chest. 1996;109:1497–502. doi: 10.1378/chest.109.6.1497. [DOI] [PubMed] [Google Scholar]
- 30.Kang SY, Sohn YH, Lee IK, Kim JS. Unilateral periodic limb movement in sleep after supratentorial cerebral infarction. Parkinson Rel Disord. 2004;10:429–31. doi: 10.1016/j.parkreldis.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Anderson KN, Bhatia KP, Losseff NA. A case of restless legs syndrome in association with stroke. Sleep. 2005;28:147–48. [PubMed] [Google Scholar]
- 32.Walters AS, Moussouttas M, Siddiqui F, et al. Prevalence of stroke in Restless Legs Syndrome: Initial Results Point to the Need for More Sophisticated Studies. Open Neurol J. 2010;4:73–77. doi: 10.2174/1874205X01004010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Walters AS, Chiuve SE, et al. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012;126:1689–94. doi: 10.1161/CIRCULATIONAHA.112.112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlesinger I, Erikh I, Avizohar O, et al. Cardiovascular risk factors in restless legs syndrome. Mov Disord. 2009;24:1587–92. doi: 10.1002/mds.22486. [DOI] [PubMed] [Google Scholar]
- 35.Cosentino FI, Aricò D, Lanuzza B, et al. Absence of cardiovascular disease risk factors in restless legs syndrome. Acta Neurol Scand. 2012;125:319–25. doi: 10.1111/j.1600-0404.2011.01563.x. [DOI] [PubMed] [Google Scholar]
- 36.Winter AC, Schürks M, Glynn RJ, et al. Restless legs syndrome and risk of incident cardiovascular disease in women and men: prospective cohort study. BMJ Open. 2012;2(2):e000866. doi: 10.1136/bmjopen-2012-000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roman MJ, Pickering TG, Schwartz JE, et al. Relation of blood pressure variability to carotid atherosclerosis and carotid artery and left ventricular hypertrophy. Arterioscler Thromb Vasc Biol. 2001;21:1507–11. doi: 10.1161/hq0901.095149. [DOI] [PubMed] [Google Scholar]
- 38.Koo BB, Blackwell T, Ancoli-Israel S, et al. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124:1223–31. doi: 10.1161/CIRCULATIONAHA.111.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Innes KE, Selfe TK, Agarwal P. Restless legs syndrome and conditions associated with metabolic dysregulation, sympathoadrenal dysfunction, and cardiovascular disease risk: a systematic review. Sleep Med Rev. 2012;16:309–39. doi: 10.1016/j.smrv.2011.04.001. [DOI] [PubMed] [Google Scholar]