Abstract
Objective
The primary aim of this study is to describe the prevalence of select oncogenic viruses within vulvar squamous cell carcinoma (VSCC) and their association with Human Immunodeficiency Virus (HIV) status in women in Botswana, where the national HIV prevalence is the third highest in the world.
Methods/materials
A cross-sectional study of biopsy-confirmed VSCC specimens and corresponding clinical data was conducted in Gaborone, Botswana. Polymerase Chain Reaction (PCR) and Immunohistochemistry (IHC) viral testing were done for Epstein-Barr Virus (EBV), Human Papilloma Virus (HPV) strains, and Kaposi's Sarcoma Herpesvirus (KSHV), and PCR viral testing alone was done for John Cunningham Virus (JCV).
Results
HPV prevalence by PCR was 100% (39/39 35/35) among tested samples. HPV16 was the most prevalent HPV strain (82.9% by PCR, 94.7% by either PCR or IHC). KSHV prevalence by PCR had a significant association with HIV status (p = 0.013), but not by IHC (p = 0.650).
Conclusions
The high burden of HPV, specifically HPV16, in VSCC in Botswana suggests a distinct HPV profile that differs from other studied populations, which provides increased motivation for HPV vaccination efforts. Oncogenic viruses KSHV and EBV were also more prevalent in our study population though their potential role in VSCC pathology is unclear.
Keywords: human immunodeficiency virus, human papilloma virus, molecular viral testing, oncogenic viruses, vulvar squamous cell carcinoma
Introduction
Human Papilloma Virus (HPV)-related cancers are estimated to account for 5.2% of the worldwide cancer burden and are increasing in incidence (1-6). Vulvar squamous cell cancer (VSCC), which makes up 90% of vulvar malignancies and 3-5% of gynecologic malignancies, is posited to have both HPV-dependent and HPV-independent oncogenic pathways (7). Existing meta-analyses and systematic reviews of primarily polymerase chain reaction (PCR) testing of vulvar cancer specimens report the overall HPV prevalence in vulvar neoplasms to be between 24.2-65.3%, with HPV 16 and HPV 18 detected in 68-96% of HPV-positive cases (6, 8-12). There are notable differences between the HPV prevalence in VSCC in different countries and continents despite the use of arguably similar laboratory techniques, but such data from sub-Saharan Africa on vulvar cancers is not readily available (6,8-10).
Epidemiological studies show that HIV positive women, who make up a disproportionate segment of the population of sub-Saharan Africa when compared to the rest of the world, are more likely to develop vulvar cancers (13-18). HIV infection has also been shown to influence the development of a number of cancers associated with oncogenic viruses other than HPV, such as Kaposi's Sarcoma Herpesvirus (KSHV) (19). The possibility of synergy between different oncogenic viruses affecting carcinogenesis has been speculated in cancers other than VSCC in HIV positive patients (20). Identifying other oncogenic viruses associated with VSCC in HIV positive women could elucidate mechanisms by which HIV infection predisposes women to develop disease. Thus studies of oncogenic viruses in VSCCs among women in areas with a high prevalence of HIV are important for understanding if they have a distinct viral profile influencing their VSCC disease burden.
The aim of our study was to investigate the prevalence of various HPV strains and other oncogenic viruses in VSCC specimens and their association with HIV infection in Botswana, the country with the world's third highest national HIV prevalence rate among adults ages 15 - 49 at 23.0% (21).
Materials & Methods
A cross-sectional study of biopsy-confirmed VSCC specimens and corresponding clinical data was conducted in Gaborone, Botswana. Approval for the study was received from the Botswana Ministry of Health's Human Research and Development Committee, Princess Marina Hospital's Institutional Review board and University of Pennsylvania's Institutional Review Board.
Specimen and Data Collection
Archived specimens were retrospectively collected from the Botswana National Health Laboratory's database. Prospective specimens were collected from women being treated at the Princess Marina Hospital (PMH) who provided written informed consent from November 2011 – March 2012. Data on age at the time of biopsy, HIV status, CD4 counts and HIV treatment corresponding to samples were obtained from clinical and laboratory electronic and paper records. Paraffin embedded vulvar tissue blocks from each subject were obtained from the Botswana National Health Laboratory.
Inclusion criteria included specimens coming from women age 18 and older and histological diagnosis of invasive VSCC by a pathologist in Botswana (see Figures 1A and 1B, Supplemental Digital Content 1, which provide examples of the clinical presentation and histologic findings for a prospectively enrolled patient). Exclusion criteria included ambiguity of anatomical source of all histologically assessed blocks of tissue and histological diagnosis of solely any grade vulvar intraepithelial neoplasia, in situ VSCC and Bowenoid papulosis.
Figure 1. Vulvar squamous cell carcinoma (VSCC).
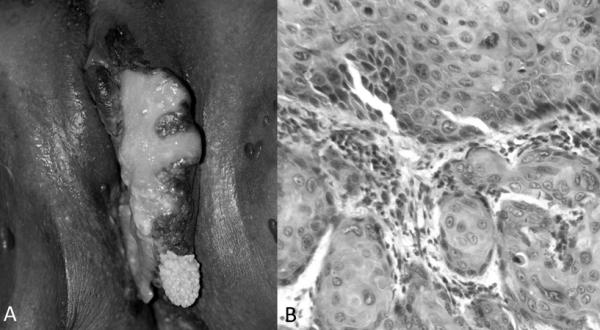
A Ulcerated plaque of the vulva consistent with VSCC. B Histopathology of vulvar biopsy with infiltration of atypical keratinocytes into the dermis consistent with invasive VSCC. (Hemotoxylin-eosin staining; original magnifications x200)
Molecular Viral Testing
PCR of EBV, HPV-L1 of multiple HPV strains (6, 7, 10, 11, 13, 14D, 16, 18, 30, 31, 32, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 54, 55, 56, 58, 59, 61, 62, 66, 72, 73) using GP5+/GP+ primers, and specific primers for HPV-6, HPV-11, HPV-16, HPV-18, HPV-31, HPV-33, and HPV-45 genomes, JCV and KSHV genomes was performed on genomic DNA isolated from paraffin sections to detect presence of viruses in the collected specimens. Immunohistochemistry (IHC) for the presence of HPV-16, EBV, and KSHV in tissues was performed on 5 μm thick paraffin- embedded sections to detect virus specific antigens within the cells of the invasive VSCC specimens. A more detailed description of our molecular viral testing methods is available as an online-only text document (see Text, Supplemental Digital Content 1, which provides information on specific primers and other specifics).
Data Analysis
All data collected was gathered in Excel and later imported into a statistical software program (Stata/SE 12.1 for Windows, StataCorp LP, College Station, TX) for consolidation, verification, and analysis. Analysis involved determination of proportions and means for all variables and execution of Fisher's exact tests for assessing association between categorical variables and Wilcoxon-Mann-Whitney tests for assessing association of categorical variables with age since the distribution of age was not normal. Subjects missing data for a given variable (e.g. HIV status, HPV 16 PCR result) were not included in analysis including said variable.
Results
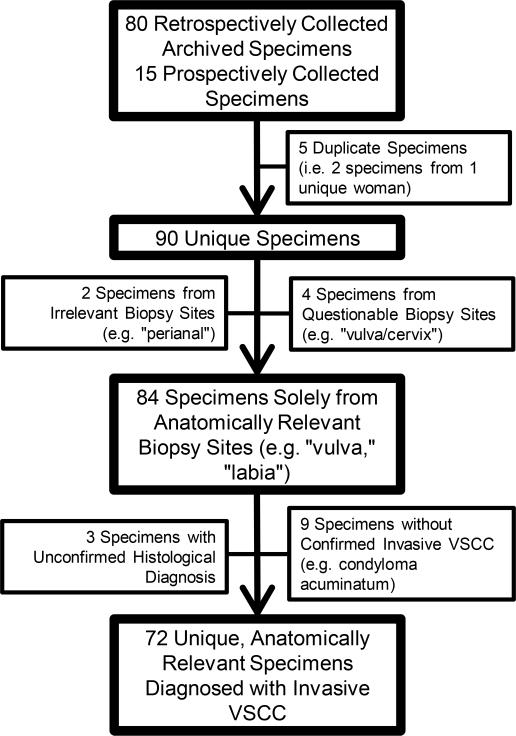
Between May 2010 and May 2012, 80 retrospective specimens, dating as far back at 1998, and 15 prospective specimens were collected. After application of the inclusion and exclusion criteria, 72 unique specimens were included in data analysis (see Figure 2).
Figure 2. Details of Study Population Determination.
Schematic representation of the application of the inclusion/exclusion criteria on collected specimens (VSCC = Vulvar squamous cell carcinoma)
Study Population Characteristics
The HIV status was known in 75.0% (54/72) subjects. 18.1% (13/72) were HIV negative and 56.9% (41/72) were HIV positive. The CD4 count was known for 47.2% (34/72) of those who were HIV positive with a range of 68 to 854 cells per cm3 of blood. 35.3% (12/34) of the collected CD4 counts were below 200 cells per cm3 of blood, which is the CD4 threshold under which HIV positive individuals are diagnosed with Acquired Immunodeficiency Syndrome (AIDS). Of the specimens for which CD4 counts were available, 20 (58.8%) had CD4 counts available before the biopsy date with a median of 242 days prior [9, 2147]. The other 14 specimens only had CD4 counts available with a median of 292 days following the biopsy date [55, 2378].
Table 1 shows the age characteristics of the study population by HIV status. The mean age at time of biopsy was 13.5 years younger for women with HIV when compared to women without HIV (p = 0.014).
Association of Oncogenic Viruses and HIV
The prevalence of oncogenic viruses by HIV status is shown in Table 2 and by CD4 counts in Table 3. KSHV presence determined by PCR was statistically different between HIV negative women compared with HIV positive women (0.0% vs 63.6%; p = 0.013). However KSHV presence determined by IHC was not statistically different between HIV negative women compared with HIV positive women (37.5% vs 54.6%; p = 0.650). The presence of all other oncogenic viruses assessed was not significantly associated with either HIV status or AIDS status (<200 vs. ≥200) (p > 0.1).
Detailed Incidence of HPV Strains
All 35 of the biopsies tested for HPV were positive for at least 1 strain. Table 4 shows the prevalence of single versus multiple HPV strains. Almost two-thirds of biopsies that underwent PCR viral testing were positive for more than one HPV strain (22/35; 62.9%). That proportion increased to almost three-quarters when accounting for multiple strain detection by PCR or IHC (26/35; 74.3%). The number of distinct HPV strains detected by PCR was not statistically different between HIV negative and HIV positive women (p = 0.737).
Among the approximately one-third of the biopsies in which only a single HPV strain was detected by PCR (13/35; 37.1%), just over two-thirds where HPV16 positive (9/13; 69.2%). 15.4% (2/13) were only positive for HPV6 by PCR and 7.9% (1/13) were positive for each HPV31 and HPV-L1 alone.
In the four biopsies that had only one HPV strain detected by PCR that was not HPV16, all of them were positive for HPV16 by IHC. Of 16 biopsies that were positive for HPV16 by PCR that underwent IHC staining, one-fourth were negative for HPV16 by IHC.
Discussion
Given that the majority of the population in Botswana is cared for in the public health sector and that Botswana National Health Laboratory and Princess Marina Hospital are the national referral laboratory and national referral hospital respectively, the study's catchment was naturally reflective of the entire nation. The sample size, though small due to the rarity of VSCC, is similar to those in other VSCC case series. To the author's knowledge, this is the first study investigating the oncogenic viral profile of VSCC in a country in which HIV highly prevalent.
Human Papilloma Virus
HPV16 prevalence by PCR was notably high at 82.9% by PCR as compared to the reported HPV16 prevalence of 29.3-50.0% in VSCCs worldwide by PCR in primarily fixed biopsies (6, 8, 12). Though three of the other high-risk HPV strains assessed—HPV31, HPV33, and HPV45—were not found independent of HPV16, their prevalence was also notably higher than those reported worldwide at 51.4%, 11.4%, and 37.1% in comparison to 0.8-1.7%, 3.3-3.5, and 0.2-1.0% (6,8). The prevalence of HPV18 in our study population (8.6%) was similar to those reported worldwide at 3.9-8.0% (6,8). The rate of multiple HPV strain infection (62.9% by PCR alone) for the cohort was also higher than other vulvar cancer studies, in which rates were reported as 2.8-9.5% (9-12).
One likely explanation for the disparities between the HPV prevalence in Botswana and other populations is that there could be a higher ratio of HPV-dependent to HPV-independent VSCCs in Botswana. Determining with more certainty the role of different HPV strains in carcinogenesis in the vulva through more specific IHC and genomic analysis could be very helpful for informing discussions on prevention through vaccination (11).
KSHV
KSHV was the only oncogenic virus in our study that demonstrated a statistically significant association with HIV status, but only with its presence as detected by PCR and not when detected by IHC. The IHC result in our sample could be due to weak sensitivity from the LANA antibody used from supernatant, which is strong with our cell line studies. It could also be due to the issue of tissue integrity or to decreased sensitivity of the antibody used for detecting the specific KSHV strain(s) in Botswana.
Our PCR findings are similar to those of a study in the United States of 30 vulvar cancer samples from HIV negative patients that were all negative for KSHV by PCR (22). The detection of KSHV via IHC among HIV negative women in our study however suggests that the virus can be found in this population. Given that the detection of KSHV by PCR and serology is known to vary by country, it could also be true for IHC (23).
Epstein-Barr Virus
The prevalence of EBV in our study was notably high at 74.3% (26/35) by PCR and 79.0% (15/19) by IHC. Other studies have shown lower rates of EBV prevalence rates of 12.5% (1/8) of VSCC cases and 26.5% (9/34) of vulvar lichen sclerosis cases, which is associated with the HPV-independent oncogenic pathway, also using PCR (24, 25). Since EBV seems to be more associated with the HPV-independent pathways based on the literature, its higher prevalence in our study population suggest that despite the high prevalence of HPV, other etiologies for VSCC could be contributing to the burden of disease in Botswana.
John Cunningham Virus
The lack of detection of JC is not surprising given that there is no data suggesting its role in VSCC.
Limitations
The greatest limitation of this study is the limited number of VSCC specimens available to be analyzed and thus its limited power. This was due in part to the rarity of VSCC and in part to the difficulty of diagnosing the condition in the population secondary to challenges such as access to health care.
The other challenge was quality and quantity of medical data available. For a quarter of specimens (18/72; 25.0%), corresponding HIV status and CD4 counts could not be determined despite extensive review of all available patient records and lab results, particularly for older retrospective specimens. Among those whose HIV status was available in the medical record, 40.7% (22/54) could not be confirmed prior to the biopsy (i.e. first record of HIV positive status was after the time of the biopsy). When only considering data for those whose HIV status at the time of biopsy was determined, 31.3% (10/32) were HIV negative and 68.8% (22/32) were HIV positive. The prevalence of KSHV by PCR dropped from 63.6% (7/11) of HIV positive women to 55.6% (5/9) but its difference from the prevalence in HIV negative women still was statistically significant (p = 0.034). The amount of data available for analysis was also limited because not all samples underwent PCR and IHC testing secondary to budgetary and workforce constraints.
Another important limitation is that PCR and even IHC viral detection is not the most conclusive manner in which to implicate an oncogenic virus in carcinogenesis and therefore can lead to an overestimation of the association between the virus and the cancer being studied (11). There is always the risk of cross-contamination with specimens, though the concern for this in our study is low given that each tissue was cut separately by sterile blades and DNA extraction performed in sterile conditions. The PCR was done in a separate area of the laboratory with appropriate controls and precaution for contamination such as using sterile tips and pipettes, and changing tips each time to avoid cross-contamination.
Future Directions
Despite its shortcomings, this study demonstrates the distinctiveness of the VSCC viral milieu in Botswana, which could influence the impact of initiatives such as HPV vaccination on the morbidity and mortality related to the disease. It also suggests that other countries, particularly those while a high HIV burden, also may have a unique oncogenic context. Further work needs to be done to understand if the detection of these various oncogenic viruses has implications for prognosis and treatment of VSCC.
Supplementary Material
Acknowledgments
Funding Sources: Supported by a pilot grant from the Penn Center for AIDS Research of University of Pennsylvania (Carrie Kovarik, MD), an International Clinical Research Fellowship grant from the Doris Duke Charitable Foundation to the University of Pennsylvania (Martha Tesfalul), and student support from the Eugene A. Stead Scholarship Committee of Duke University (Chikoti M. Wheat).
Footnotes
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- 1.Tota JE, Chevarie-Davis M, Richardson LA, et al. Epidemiology and burden of HPV infection and related diseases: Implications for prevention strategies. Prev Med. 2001;53:S12–S21. doi: 10.1016/j.ypmed.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Lowy DR, Schiller JT. Reducing HPV-Associated Cancer Globally. Cancer Prev Res. 2012;5:18–23. doi: 10.1158/1940-6207.CAPR-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baandrup L, Varbo A, Munk C, et al. In situ and invasive squamous cell carcinoma of the vulva in Denmark 1978-2007-a nationwide population-based study. Gynecol Oncol. 2011 Jul;122(1):45–9. doi: 10.1016/j.ygyno.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Bodelon C, Madeleine MM, Voight LF, et al. Is the incidence of invasive vulvar cancer increasing in the United States? Cancer Causes Control. 2009 Nov;20(9):1779–82. doi: 10.1007/s10552-009-9418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011 doi: 10.1016/S0140-6736(11)61351-2. DOI:10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 6.Smith JS, Backes DM, Hoots BE, et al. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet Gynecol. 2009 Apr;113(4):917–24. doi: 10.1097/AOG.0b013e31819bd6e0. [DOI] [PubMed] [Google Scholar]
- 7.Ueda Y, Enomoto T, Kimura T, et al. Two distinct pathways to development of squamous cell carcinoma of the vulva. J Skin Cancer. 2011;2011:951250. doi: 10.1155/2011/951250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre) Summary Report. Barcelona, Spain: Sep 15, 2010. Human Papillomavirus and Related Cancers: Africa. [Google Scholar]
- 9.De Vuyst H, Clifford GM, Nascimento MC, et al. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009 Apr 1;124(7):1626–36. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 10.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008 Nov 15;113(10 Suppl):3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Nieuwenhof HP, van Kempen LC, de Hullu JA, et al. The etiologic role of HPV in vulvar squamous cell carcinoma fine tuned. Cancer Epidemiol Biomarkers Prev. 2009 Jul;18(7):2061–7. doi: 10.1158/1055-9965.EPI-09-0209. [DOI] [PubMed] [Google Scholar]
- 12.Insignia RP, Liaw K, Johnson LG, et al. A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:1611–1622. doi: 10.1158/1055-9965.EPI-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JE, Sunborg MJ, Kost E, et al. Vulvar cancer in human immunodeficiency virus-seropositive premenopausal women: a case series and review of the literature. J Low Genit Tract Dis. 2005 Jan;9(1):7–10. doi: 10.1097/00128360-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi AK, Madeleine MM, Biggar RJ, et al. Risk of Human Papillomavirus– Associated Cancers Among Persons With AIDS. J Natl Cancer Inst. 2009;101(16):1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conley LJ, Ellerbrock TV, Bush TJ, et al. HIV-1 infection and risk of vulvovaginal and perianal condylomata acuminata and intraepithelial neoplasia: a prospective cohort study. Lancet. 2002 Jan 12;359(9301):108–13. doi: 10.1016/S0140-6736(02)07368-3. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson DJ, Paramsothy P, Cu-Uvin S, et al. HIV Epidemiology Research Study Group. Vulvar, vaginal, and perianal intraepithelial neoplasia in women with or at risk for human immunodeficiency virus. Obstet Gynecol. 2006 May;107(5):1023–8. doi: 10.1097/01.AOG.0000210237.80211.ff. [DOI] [PubMed] [Google Scholar]
- 17.Massad LS, Silverberg MJ, Springer G, et al. Effect of antiretroviral therapy on the incidence of genital warts and vulvar neoplasia among women with the human immunodeficiency virus. Am J Obstet Gynecol. 2004 May;190(5):1241–8. doi: 10.1016/j.ajog.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg MJ, Chao C, Leyden WA, et al. HIV Infection and the risk of cancers with and without a known infectious cause. AIDS. 2009 Nov 13;23(17):2337–2345. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene W, Kuhne K, Ye F, et al. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat Res. 2007;133:69–127. doi: 10.1007/978-0-387-46816-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simbiri KO, Murakami M, Feldman M, et al. Multiple oncogenic viruses identified in Ocular surface squamous neoplasia in HIV-1 patients. Infectious Agents and Cancer. 2010;5:6. doi: 10.1186/1750-9378-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report: UNAIDS Report on the Global AIDS Epidemic: 2013. UN Joint Programme on HIV/AIDS; Geneva, Switzerland: 2013. [Google Scholar]
- 22.Tasaka T, Said JW, Morosetti R, et al. Is Kaposi's sarcoma--associated herpesvirus ubiquitous in urogenital and prostate tissues? Blood. 1997 Mar 1;89(5):1686–9. [PubMed] [Google Scholar]
- 23.Capuano M, La Porola IL, Cattani P, et al. Re: Kaposi's sarcoma associated herpesvirus deoxyribonucleic acid sequences: lack of detection in prostatic tissue of human immunodeficiency virus-negative immunocompetent adults. J Urol. 1998 Aug;160(2):506–6. doi: 10.1016/s0022-5347(01)62942-2. [DOI] [PubMed] [Google Scholar]
- 24.Cheung AN, Khoo US, Kwong KY, et al. Epstein-Barr virus in carcinoma of the vulva. J Clin Pathol. 1993 Sep;46(9):849–51. doi: 10.1136/jcp.46.9.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aidé S, Lattario FR, Almeida G, et al. Epstein-Barr virus and human papillomavirus infection in vulvar lichen sclerosus. J Low Genit Tract Dis. 2010 Oct;14(4):319–22. doi: 10.1097/LGT.0b013e3181d734f1. [DOI] [PubMed] [Google Scholar]
- 26.Garland SM, Insinga RP, Sings HL, et al. Human papillomavirus infections and vulvar disease development. Cancer Epidemiol Biomarkers Prev. 2009 Jun;18(6):1777–84. doi: 10.1158/1055-9965.EPI-09-0067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.