Abstract
The objective of this research was to clarify the aging-related changes in in vitro-matured bovine oocytes. Firstly, we examined the fertilization and embryonic development of bovine oocytes after 22 and 30–34 h of in vitro maturation (IVM). The oocytes after 30–34 h of IVM (penetrated by sperm at around 40 h after starting IVM) showed a lower developmental rate to blastocysts (P<0.01), although normal fertilization rates were similar regardless of IVM duration. In the next experiment, reactive oxygen species (ROS), mitochondrial activity and ATP content in oocytes after 20, 30 and 40 h of IVM were examined. The lowest level of ROS was found in the group subjected to 30 h of IVM. The mitochondrial activity and ATP content in the group subjected to 40 h of IVM were higher than in the group subjected to 20 h of IVM (P<0.01), and those in the group subjected to 30 h of IVM showed intermediate values. Thereafter, the mitochondrial activities at 3 days after in vitro fertilization in embryos derived from the oocytes subjected to 22 and 34 h of IVM were evaluated. In the group subjected to 34 h of IVM, high-polarized mitochondria were frequently observed at the periphery of blastomeres. The present results suggest that high mitochondrial activity observed in oocytes after prolonged IVM culture and localization of high-polarized mitochondria at the periphery of blastomeres during early embryonic development may be associated with the low developmental competence in aged bovine oocytes.
Keywords: Aging, ATP, Bovine oocytes, Mitochondria, ROS
In humans and domestic animals, it is well known that postovulatory aging of oocytes at the metaphase II (M-II) stage adversely affects the outcome of assisted reproductive technologies (ART), such as artificial insemination [1], in vitro fertilization (IVF) [2, 3] and intracytoplasmic sperm injection [4, 5]. In humans and rodents, postovulatory aging of oocytes is defined as numerous morphological and cellular alterations and causes a decrease in fertilization and embryonic development, which has been previously reviewed [6, 7]. In bovines, both in vivo and in vitro aging of oocytes cause a decrease in fertilization and embryonic development [1, 2, 8,9,10,11]. However, there are few data on aging-related changes in bovine oocytes. Research on aging in bovine oocytes will contribute to the development of a method for preventing aging in in vitro-matured bovine oocytes and eventually to improvement of ART efficiency.
Recently, studies on murine oocytes have indicated the possibility that oxidative stress acts as a trigger for a cascade of several events associated with oocyte aging and that one of the aging-related changes caused by oxidative stress is mitochondrial dysfunction [6]. It has been reported that the aging of murine oocytes causes increased oxidative stress [12], mitochondrial dysfunction [13, 14] and decreased intracytoplasmic levels of ATP [15]. In in vitro-matured bovine oocytes, the changes in level of oxidative stress [16], mitochondrial activity [17, 18] and ATP content [19,20,21,22] during in vitro maturation (IVM) culture for less than 24 h have been determined, but there is little data on the changes in these parameters associated with bovine oocyte aging. Therefore, it is still unclear whether the extension of IVM duration in bovine oocytes causes the aging-related changes in oxidative stress, mitochondrial activity and ATP content.
In previous studies, bovine oocytes at about 30 h after IVM were treated as aged or slightly aged oocytes and used to investigate aging-related changes [9, 23, 24]. This was because the oocytes after 30 h of IVM showed a low developmental rate to blastocysts [9]. However, after 30 h of IVM, degradation of the microfilament-rich domain overlying the spindle in bovine oocytes was not observed, which had been observed in porcine aged oocytes [24]. Moreover, maturation-promoting factor (MPF) activity was similar to that in bovine oocytes matured for 24 and 32 h [9], although a decrease in activity of MPF in bovine oocytes matured for 40 h was observed [25]. Since it takes several hours for bovine oocytes to be penetrated by sperm after starting IVF [9, 26], studies on the characteristics of aged oocytes should not be based on developmental competence corresponding to the duration of IVM, but instead should be based on the timing of sperm penetration.
The present study was conducted to clarify the aging-related changes related to oxidative stress, mitochondrial activity and ATP content in in vitro-matured bovine oocytes and to mitochondrial activity in embryos at around the 8-cell embryo stage derived from in vitro-fertilized bovine oocytes. Firstly, we confirmed the competence of fertilization and embryonic development in oocytes subjected to 22 and 30–34 h of IVM. We then examined reactive oxygen species (ROS), mitochondrial activity and ATP content after 20, 30 and 40 h of IVM culture and examined the mitochondrial activity in embryos at 72 h after IVF.
Materials and Methods
Chemicals
All the chemicals and reagents used for this study were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise stated.
In vitro maturation and fertilization of bovine oocytes
IVM was performed as previously described [27]. Briefly, bovine ovaries (mostly Holstein breed) obtained at a local abattoir were kept in plastic bags at 20 C and were transported to the laboratory within 6–10 h of collection. Bovine cumulus-oocyte complexes (COCs) were aspirated from small antral follicles (2 to 8 mm in diameter). The COCs with brown-colored ooplasm surrounded by intact cumulus investments [28] were washed twice in HEPES-buffered Tyrode’s medium [29] supplemented with 3 mg/ml bovine serum albumin (BSA, fraction V), 0.2 mM sodium pyruvate and 50 μg/ml gentamicin sulfate. The COCs were then cultured for 20 to 40 h under a humidified atmosphere of 5% CO2 in air at 39 C in droplets of IVM medium (about 10 COCs/50 μl). IVM medium was composed of HEPES-buffered TCM-199 (Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal calf serum (FCS, Invitrogen), 0.02 units/ml follicle stimulating hormone (from porcine pituitary), 1 μg/ml estradiol-17β, 0.2 mM sodium pyruvate and 50 μg/ml gentamicin sulfate.
IVF was conducted using frozen-thawed semen from one Holstein bull according to a procedure described previously [30] with slight modifications. In brief, motile sperm (2 × 106 sperm/ml) separated from thawed semen using a Percoll (GE Healthcare, Buckinghamshire, UK) gradient (45 and 90%) were co-incubated with COCs in droplets of IVF medium (about 10 COCs/100 μl). IVF medium was composed of modified Brackett and Oliphant isotonic medium [26] containing 3 mg/ml fatty acid-free BSA, 2.5 mM theophylline, 20 μM penicillamine, 10 μM hypotaurine and 1 μM epinephrine. Co-incubation of COCs and sperm was performed for 18 h under 5% CO2, 5% O2 and 90% N2 at 39 C.
Evaluation of the fertilized oocytes
After 18 h of IVF, oocytes were freed from cumulus cells by vortexing. Denuded oocytes were fixed with ethanol:acetic acid (3:1) and stained with a 1% aceto-orcein solution. Their fertilization statuses (sperm penetration and pronuclear formation) were examined under a phase-contrast microscope [19, 28]. Oocytes having an enlarged sperm head(s) or male pronucleus(ei) were defined as penetrated by sperm, and the following categories of oocytes penetrated by sperm were recorded: 1) oocytes with male and female pronuclei or with an enlarged sperm head and anaphase II/telophase II chromosome (normal fertilization), 2) oocytes with more than two enlarged sperm heads or male pronuclei (polyspermy) and 3) oocytes with an enlarged sperm head and female pronucleus or with male pronucleus and telophase II chromosome (asynchronous fertilization).
In vitro culture and evaluation of subsequent embryonic development
To determine the developmental competence of oocytes, inseminated oocytes were assigned to in vitro culture (IVC) according to a procedure described previously [27, 31]. In brief, inseminated oocytes were freed from cumulus cells by vortexing at 18 h post insemination (hpi). Denuded oocytes were washed three times and cultured for 6 days under 5% CO2, 5% O2 and 90% N2 at 39 C in droplets of IVC medium (25–30 oocytes/30 μl). IVC medium was a modified synthetic oviduct fluid containing 1 mM glutamine, 12 essential amino acids for Basal Medium Eagle, 7 nonessential amino acids for Minimum Essential Medium and 10 µg/ml insulin and further supplemented with 5 mM glycine, 5 mM taurine, 1 mM glucose and 3 mg/ml fatty acid-free BSA. After 2 and 7 days of IVF (44 to 48 and 166 to 170 hpi, respectively), cleavage and development to the blastocyst stage were assessed, respectively. All blastocysts were subjected to counting of the total number of cells by an air-drying method [32].
Measurement of ROS in individual oocytes
The quantity of H2O2 produced by individual oocytes was measured as the level of ROS according to a previous report [33]. ROS in oocytes can be quantified by measuring 2’,7’-dichlorofluorescein diacetate (DCF) [34]. DCF fluorescence is generated by H2O2 from 2’,7’-dichlorodihydrofluorescein, which is formed by intracellular esterase from 2’,7’-dichlorodihydrofluorescein diacetate (DCHFDA).
After IVM culture, oocytes were freed from the cumulus cells by vortexing. Denuded oocytes with a polar body were incubated with 10 μM DCHFDA in Dulbecco’s phosphate-buffered saline (DPBS) supplemented with 10% FCS for 15 min at 39 C and washed in DPBS. Stained oocytes were transferred to a slide glass with a small amount of DPBS and pressed gently with a cover slide. Their fluorescence emissions were then observed under a fluorescence microscope using an appropriate filter (BZ-9000; Keyence, Osaka, Japan), and the sectioned fluorescent images of each oocyte were acquired at 2 μm intervals. The mean green fluorescent intensity of each oocyte, which represents the H2O2 level, was calculated by analysis software (BZ-H2A, Keyence).
Evaluation of mitochondrial activity in individual oocytes and embryos
Denuded oocytes or embryos at 72 hpi were stained with JC-1 (Cell Technology Inc., Mountain View, CA, USA) as described previously [18] with slight modifications. JC-1 is a fluorescent dye that accumulates in mitochondria and shows the membrane potential across the matrix membrane [35]. JC-1 fluorescence has two emission peaks, with red fluorescence (JC-1 dimers) indicating high-polarized mitochondria (high membrane potential) and green fluorescence (JC-1 monomers) indicating low-polarized mitochondria (low membrane potential) [35]. Mitochondrial activity can be evaluated by the intensity of the red/green fluorescence [13, 17, 18].
Briefly, denuded oocytes or embryos were incubated with 1 μM JC-1 and 1 μg/ml Hoechst 33342 in DPBS supplemented with 10% FCS for 15 min at 37 C and washed twice in DPBS. Stained oocytes or embryos were transferred to a slide glass with a small amount of DPBS and pressed gently with a cover slide. They were then observed under a fluorescence microscope (BZ-9000). The distributions of JC-1 dimers with red fluorescence and monomers with green fluorescence were detected using the red filter and green filter of the microscope, respectively. Mitochondrial activity of the oocytes at the M-II stage or cleaved oocytes (evaluated by Hoechst staining) was determined by the intensity of the red/green fluorescence using software (BZ-H2A).
Measurement of ATP content in individual oocytes
After IVM culture, oocytes were freed from the cumulus cells by vortexing. The ATP content of individual oocytes with a polar body was measured according to a previous report [19, 36]. Briefly, a denuded oocyte was washed four times in the sample buffer and transferred to 25 μl of the sample buffer in a 1.5 ml tube. The sample buffer consisted of 99.0 mM NaCl, 3.1 mM KCl, 0.35 mM NaH2PO4, 21.6 mM sodium lactate, 10.0 mM HEPES, 2.0 mM CaCl2, 1.1 mM MgCl2, 25.0 mM NaHCO3, 1.0 mM sodium pyruvate, 0.1 mg/ml of gentamicin sulfate and 6.3 mg/ml of BSA [36]. These tubes were placed in boiling water for 3 min to inactivate the endogenous phosphatases and then frozen at −80 C until assay. All assay reagents were purchased as a kit (ATP bioluminescent somatic cell assay kit, FL-ASC) and prepared according to the manufacturer’s instructions. The ATP stock solution was diluted to concentrations of 0.16 to 10 pmol/25 μl in sample buffer for the ATP standards. The ATP standards and samples in 1.5 ml tubes were kept on ice, and 50 μl of ice-cold somatic cell-releasing agent was added to all tubes. After the tubes were kept on ice for 5 min, the contents of the tubes were transferred to a white 96-well plate (Labsystems, Tokyo, Japan). Thereafter, 100 μl of assay mix was added to each well at 5 sec intervals and held at room temperature for 5 min to pass through the initial chemiluminescence flash period. ATP content in an oocyte was quantified by measuring the luminescence (Luminescensor JNR AB-2100, Atto, Tokyo, Japan).
Experimental design
In Experiment 1, bovine oocytes after 22 and 30–34 h of IVM were assigned for 18 h of IVF, and their fertilization statuses were determined. In addition, the percentages of cleavage and development to the blastocyst stage were evaluated. Out of 473 oocytes subjected to IVM and IVF, 351 oocytes were subjected to IVC (20–30 oocytes/replicate), and the remaining 122 oocytes were used for the fertilization evaluation (10–13 oocytes/replicate).
In the preliminary study, we examined the percentages of oocytes penetrated by sperm at 4, 8 and 12 h after starting IVF in bovine oocytes subjected to IVM for 22 or 30 h, and the times when more than 50% of oocytes were penetrated by sperm were estimated at 6 to 8 h after starting IVF (data not shown). Therefore, in Experiment 2, oocytes subjected to IVM for 20 h (immediately after M-II arrival [19]), 30 and 40 h were used for determination of ROS, mitochondrial activity and ATP content. The 30 and 40 h time points for IVM were considered the times when sperm penetration occurred in oocytes subjected to 22 and 30–34 h of IVM, respectively. For ROS measurement, a total of 78 oocytes were subjected to IVM, and oocytes with a polar body clearly observed under a stereomicroscope were used for the experiment (10–13 oocytes/replicate). Also, for evaluating mitochondrial activity and ATP content, a total of 216 oocytes were subjected to IVM, and oocytes with a polar body clearly observed under a stereomicroscope were used for the experiment (9–14 oocytes/replicate). In addition, oocytes were then subjected to 22 h (penetrated by sperm at around 30 h after starting IVM) and 34 h (penetrated by sperm at around 40 h after starting IVM) of IVM, IVF and IVC, and the cleavage and developmental stage of embryos were confirmed by the number of nuclei stained with Hoechst 33342 at 72 hpi. The mitochondrial activities of cleaved embryos were then examined. The distribution of high-polarized mitochondria in embryos was also examined, and the percentage of embryos with high-polarized mitochondria at the periphery of blastomeres was recorded. In the experiment, 20 and 21 embryos derived from the oocytes subjected to 22 and 34 h of IVM were used (1 replicate).
Statistical analysis
Data for fertilization and embryonic development (Experiment 1) and mitochondrial activity of embryos at 3 days after IVF (Experiment 2) were analyzed by Student’s t-test. ROS, mitochondrial activity and ATP content of oocytes after IVM culture (Experiment 2) were analyzed using one-way analysis of variance followed by Tukey-Kramer’s honestly significant different test as a post hoc test. Data for embryonic development and distribution of high-polarized mitochondria in embryos were analyzed by Fisher’s exact test (Experiment 2). The level of statistical significance was set at P<0.05. Statistical analyses were performed using JMP version 10.0.2 (SAS Institute, Cary, NC, USA).
Results
Experiment 1
The data for fertilization are shown in Table 1. The percentages of normal fertilization, polyspermy and sperm penetration were similar regardless of IVM duration. However, the percentage of oocytes that formed a pronucleus asynchronously in the group subjected to 30–34 h of IVM was higher than in the group subjected to 22 h (P<0.01). In the group subjected to 30–34 h of IVM, the percentages of delay in male and female pronucleus formation were 10.4 and 1.3%, respectively. As shown in Table 2, the cleavage rate tended to be lower in the group subjected to 30–34 h of IVM than in the group subjected to 22 h of IVM (P = 0.06). The percentages of blastocysts based on inseminated and cleaved oocytes in the group subjected to 30–34 h of IVM were also lower than in the group subjected to 22 h of IVM (P<0.01). Total cell numbers in blastocysts were similar regardless of IVM duration.
Table 1. The effect of IVM duration of bovine oocytes on fertilization at 18 h after IVF.
| Duration of IVM (h) | No. of oocytes (replicates) |
% of oocytes with |
Total penetration (%) | ||
| Normal fertilization* |
Polyspermy | Asynchronous fertilization** |
|||
| 22 | 45 (4) | 84.6 ± 8.4 | 8.6 ± 9.2 | 0.0 ± 0.0a | 93.2 ± 8.7 |
| 30–34 | 77 (7) | 79.1 ± 13.7 | 9.2 ± 10.5 | 11.7 ± 6.9b | 100 ± 0.0 |
a,b Values (mean ± SD) with different superscripts within columns are significantly different (P<0.01). * Normal fertilization: male and female pronuclei or an enlarged sperm head and anaphase II/telophase II chromosome. ** Asynchronous fertilization: an enlarged sperm head and female pronucleus or male pronucleus and telophase II chromosome.
Table 2. The effect of IVM duration of bovine oocytes on embryonic development at 2 and 7 days after IVF.
| Duration of IVM (h) | No. of embryos (replicates) |
% ≥2 cells/ inseminated |
% blastocysts/ inseminated |
% blastocysts/ cleaved |
Total cell no.in blastocysts (n) |
| 22 | 161 (6) | 81.1 ± 5.6A | 51.6 ± 9.4a | 64.1 ± 13.1a | 172.5 ± 77.5 (83) |
| 30–34 | 190 (7) | 71.1 ± 10.6B | 24.2 ± 8.8b | 33.8 ± 11.4b | 157.6 ± 56.5 (45) |
a,b Values (mean ± SD) with different superscripts within the same column differ significantly (P<0.01). A,B Values (mean ± SD) with different superscripts within the same column tended to be different (P = 0.06).
Experiment 2
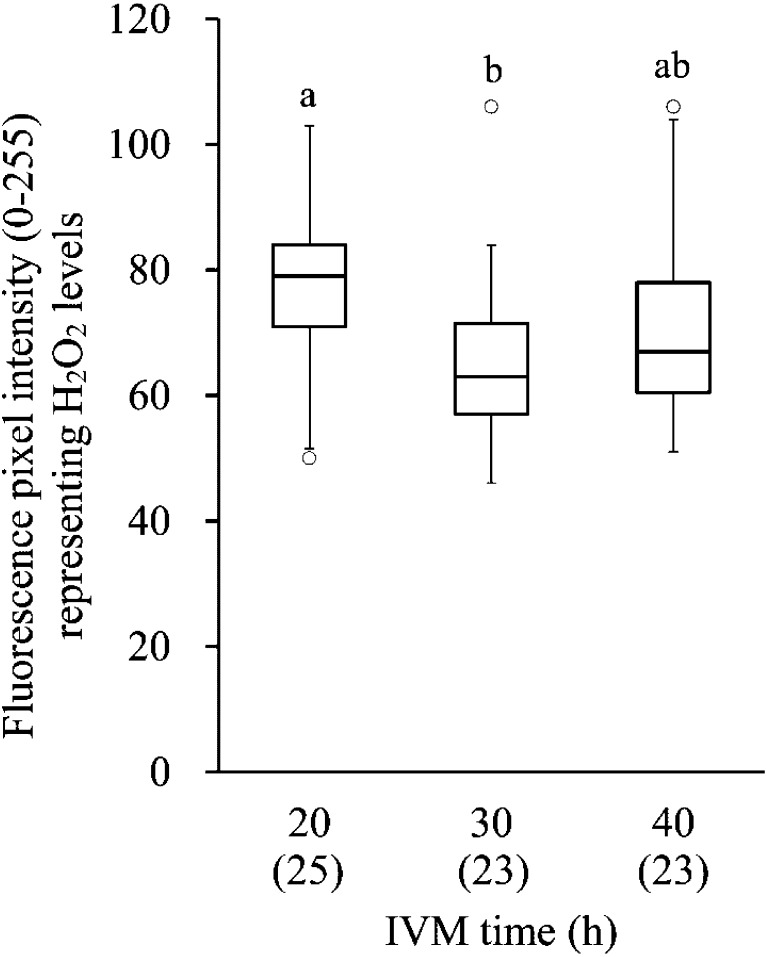
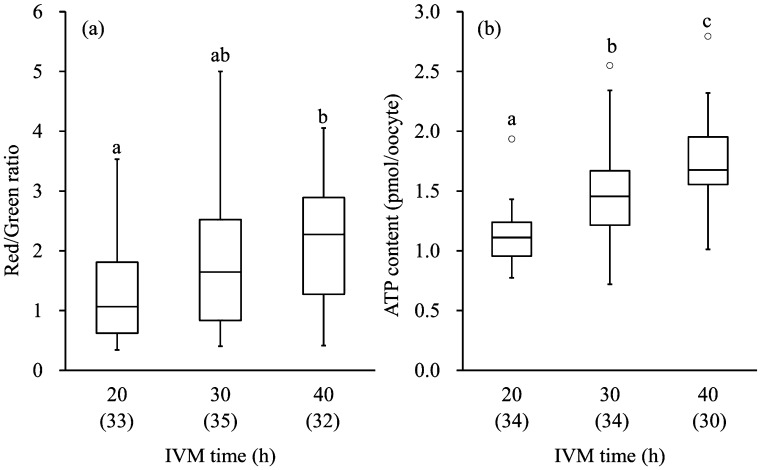
The levels of ROS production in oocytes after different IVM culture periods are shown in Fig. 1. Mean intensity of DCF fluorescence in the group subjected to 40 h of IVM was similar to that of the groups subjected to 20 and 30 h of IVM, and that in the group subjected to 30 h of IVM was lower than that in the group subjected to 20 h of IVM (P<0.01). The mitochondrial activity and the ATP content in oocytes of different IVM culture periods are shown in Fig. 2. The mitochondrial activity in the group subjected to 40 h of IVM was higher than in the group subjected to 20 h of IVM (P<0.01). The mitochondrial activity in the group subjected to 30 h of IVM showed an intermediate value between the groups subjected to 20 and 40 h of IVM. The ATP content in the group subjected to 40 h of IVM was higher than in the other groups (P<0.01).
Fig. 1.
Box plots of the mean green intensity of DCF fluorescence in oocytes after IVM culture, which represents the H2O2 level. The lower and upper ± 1.5 quartiles are indicated by whiskers, the lower and upper ends of the boxes indicate the 25th and 75th quartiles, and the line across the middle of the box identifies the median sample value. The circles represent the outliers. a,b Values with different characters differ significantly among the three IVM groups (P<0.01). Numbers of oocytes used are indicated in parentheses (2 replicates).
Fig. 2.
Box plots of mitochondrial activity (a) and ATP content (b) in oocytes after IVM culture. The lower and upper ± 1.5 quartiles are indicated by whiskers, the lower and upper ends of the boxes indicate the 25th and 75th quartiles, and the line across the middle of the box identifies the median sample value. The circles represent the outliers. a,b,c Values with different characters differ significantly among the three IVM groups (P<0.01). Numbers of oocytes used are indicated in parentheses (3 replicates).
At 72 hpi, the percentage of ≥8-cell stage embryos in the group subjected to 22 h of IVM (60.0%) was higher than in the group subjected to 34 h of IVM (23.8%) (P<0.05). As shown in Fig. 3, the mitochondrial activity of embryos at 72 hpi was similar regardless of IVM duration; however, the percentage of embryos having high-polarized mitochondria at the periphery of blastomeres (Fig. 4) was higher in the group subjected to 34 h of IVM (81.0%) than in the group subjected to 22 h of IVM (30.0%) (P<0.01).
Fig. 3.
Mitochondrial activity in ≥2-cell embryos derived from the oocytes subjected to 22 and 34 h of IVM. The lower and upper ± 1.5 quartiles are indicated by whiskers, the lower and upper ends of the boxes indicate the 25th and 75th quartiles, and the line across the middle of the box identifies the median sample value. Numbers of oocytes used are indicated in parentheses (1 replicate).
Fig. 4.
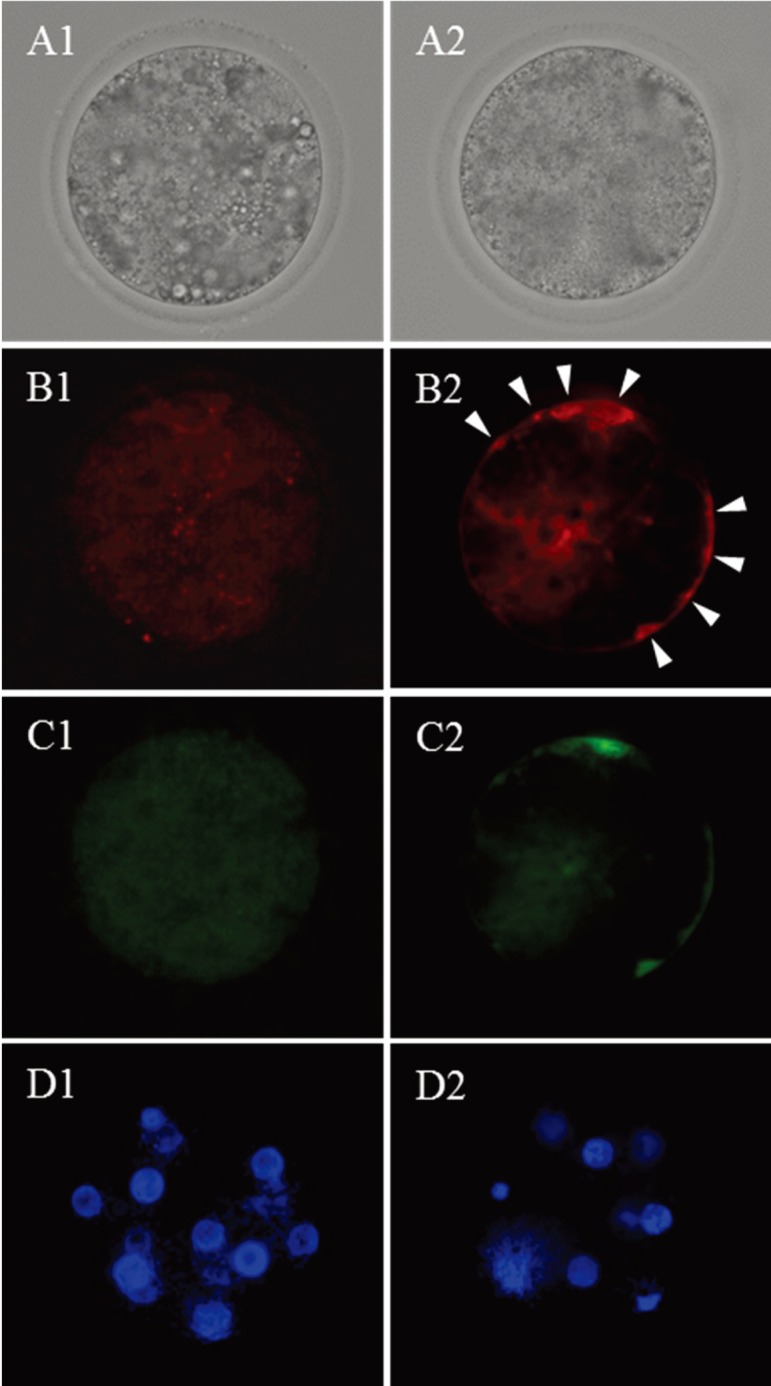
Bright field (A) and fluorescent micrographs (B, C and D) of embryos (72 hpi) derived from the oocytes subjected to 22 (1) and 34 h (2) of IVM culture. B, high-polarized mitochondria stained by JC-1; C, low-polarized mitochondria stained by JC-1; D, nuclei stained by Hoechst 33342. Twelve and 8 nuclei are observed in D1 and 2, respectively. The embryo derived from the oocyte subjected to 22 h of IVM shows high-polarized mitochondria at the periphery of blastomeres (white arrows: B2).
Discussion
In the present study, normal fertilization was similar regardless of IVM duration, but developmental competence to the blastocyst stage was low in the bovine oocytes subjected to 30–34 h of IVM. These results were consistent with the results of previous studies [2, 9,10,11]. Moreover, asynchronous fertilization has been frequently observed in bovine oocyte subjected to 30–34 h of IVM. The delay in male pronucleus formation might be due to the deficiency of male pronucleus growth factor in the group subjected to 30–34 h of IVM [37, 38]. The reason for the delay in female pronucleus formation is unclear, but a similar delay in female pronucleus formation was observed in fertilized oocytes from aged hamsters [39].
In contrast to the past report that the level of cytoplasmic ROS in oocytes increased with oocytes aging in murine and swine [12, 40, 41], extension of IVM culture up to 40 h did not cause the increased level of cytoplasmic ROS in the present study. Moreover, although it has been reported that mitochondrial activity and ATP contents in human, murine and porcine oocytes decrease with aging [13,14,15, 42, 43], the mitochondrial activity and ATP content in bovine oocytes subjected to 40 h of IVM was highest among all IVM durations in the present study. Therefore, it is thought that the decrease in developmental competence in oocytes after prolonged IVM culture was not due to the mitochondrial dysfunction and decrease in intracytoplasmic levels of ATP caused by oxidative stress. On the other hand, ROS in the oocytes after 30 h of IVM was lower than in the oocytes after 20 h of IVM, and this result showed that the oocytes after 30 h of IVM had high competence to protect themselves from oxidative stress. Therefore, it is thought that one of the reasons for this high developmental competence of oocytes subjected to 22 h of IVM (penetrated by sperm at around 30 h after the initiation of IVM) was due to the low oxidative stress in oocytes resulting from their high competence to protect themselves from oxidative stress. In a previous study [12], it was reported that cumulus cells prevented the increase of ROS during in vitro aging. Although we used only oocytes having complete cumulus investments, the relationship between the function of cumulus cells and ROS generation during IVM culture should be examined in further study.
Van Blerkom and Davis [44] indicated that the subplasmalemmal domains, including high-polarized mitochondria, were extruded as fragments from human embryo blastomeres and that this phenomenon caused failure to cleave during early embryonic development. In the present study, high-polarized mitochondria were frequently observed at the periphery of blastomeres in embryos derived from oocytes with extended IVM culture. These high-polarized mitochondria at the periphery of blastomeres might have already been extruded from blastomeres like in the previous report [44], and it is possible that the extrusion of high-polarized mitochondria from blastomeres causes lower early embryonic development of oocytes with extended IVM culture. However, in the present study, we were unable to confirm the clear extrusion of high-polarized mitochondria from blastomeres because the bovine embryos contained a large number of lipid droplets in their ooplasm, unlike human embryos [45], and it was necessary to press the embryos with a cover slide to observe the mitochondria stained by JC-1. In future study, we should examine the localization of high-polarized mitochondria in embryos derived from oocytes with extended IVM culture in detail by electron microscope analysis.
Although the present study does not clarify the direct causal relationship between the enhanced mitochondrial activity and low developmental competence of bovine oocytes, the present results suggest that enhanced mitochondrial activity may be one of the reasons for low developmental competence of bovine oocytes after an extended duration of IVM culture. High-polarized mitochondria in oocytes are associated with elevated levels of ATP generation [46]. Tamassia et al. [21] indicated that the ATP content in ovum pick up (OPU)-derived oocytes did not increase during IVM culture but that the ATP content in abattoir-derived oocytes did increase. Also, it has been reported that OPU-derived bovine oocytes retained high developmental competence during longer periods of IVM culture than abattoir-derived oocytes [22]. These previous reports indicate that OPU-derived oocytes can control mitochondrial activity and that this ability contributes to maintaining high developmental competence during long periods. In the present study, blastocysts derived from oocytes subjected to 30–34 h of IVM culture had a similar number of cells to those derived from oocytes subjected to 22 h of IVM culture. This suggests that some oocytes subjected to more than 30 h of IVM maintain their developmental competence. In future studies, we should examine the mechanisms of maintenance of mitochondrial activity in in vitro-matured bovine oocytes and the relationship between mitochondrial activity and developmental competence in detail.
In conclusion, the present study suggests that low developmental competence of bovine oocytes with extended IVM culture is probably not due to low mitochondrial activity or low ATP content but is probable due to high mitochondrial activity at fertilization and localization of high-polarized mitochondria at the periphery of blastomeres during early embryonic development. Enhanced mitochondrial activity seems to have detrimental effects on the developmental competence of bovine oocytes, and this is possibly related to oocyte aging in vitro. Future detailed studies on the relationship between enhanced high mitochondrial activity at fertilization and subsequent embryo development should be conducted.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (No. 25450441) from the Japan Society for the Promotion of Science to M Nagano.
References
- 1.Saacke RG, Dalton JC, Nadir S, Nebel RL, Bame JH. Relationship of seminal traits and insemination time to fertilization rate and embryo quality. Anim Reprod Sci 2000; 60–61: 663–677 [DOI] [PubMed] [Google Scholar]
- 2.Agung B, Otoi T, Wongsrikeao P, Taniguchi M, Shimizu R, Watari H, Nagai T. Effect of maturation culture period of oocytes on the sex ratio of in vitro fertilized bovine embryos. J Reprod Dev 2006; 52: 123–127 [DOI] [PubMed] [Google Scholar]
- 3.Harrison KL, Wilson LM, Breen TM, Pope AK, Cummins JM, Hennessey JF. Fertilization of human oocytes in relation to varying delay before insemination. Fertil Steril 1988; 50: 294–297 [DOI] [PubMed] [Google Scholar]
- 4.Emuta C, Horiuchi T. Effects of timing of activation and aging of bovine oocytes fertilized by intracytoplasmic sperm injection (ICSI) on cleavage and subsequent embryonic development in vitro. J Reprod Dev 2001; 47: 399–405 [Google Scholar]
- 5.Yanagida K, Yazawa H, Katayose H, Suzuki K, Hoshi K, Sato A. Influence of oocyte preincubation time on fertilization after intracytoplasmic sperm injection. Hum Reprod 1998; 13: 2223–2226 [DOI] [PubMed] [Google Scholar]
- 6.Lord T, Aitken RJ. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013; 146: R217–R227 [DOI] [PubMed] [Google Scholar]
- 7.Miao Y-L, Kikuchi K, Sun Q-Y, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update 2009; 15: 573–585 [DOI] [PubMed] [Google Scholar]
- 8.Roelofs JB, Graat EA, Mullaart E, Soede NM, Voskamp-Harkema W, Kemp B. Effects of insemination-ovulation interval on fertilization rates and embryo characteristics in dairy cattle. Theriogenology 2006; 66: 2173–2181 [DOI] [PubMed] [Google Scholar]
- 9.Rispoli LA, Lawrence JL, Payton RR, Saxton AM, Schrock GE, Schrick FN, Middlebrooks BW, Dunlap JR, Parrish JJ, Edwards JL. Disparate consequences of heat stress exposure during meiotic maturation: embryo development after chemical activation vs fertilization of bovine oocytes. Reproduction 2011; 142: 831–843 [DOI] [PubMed] [Google Scholar]
- 10.Long CR, Damiani P, Pinto-Correia C, MacLean RA, Duby RT, Robl JM. Morphology and subsequent development in culture of bovine oocytes matured in vitro under various conditions of fertilization. J Reprod Fertil 1994; 102: 361–369 [DOI] [PubMed] [Google Scholar]
- 11.Ward F, Enright B, Rizos D, Boland M, Lonergan P. Optimization of in vitro bovine embryo production: effect of duration of maturation, length of gamete co-incubation, sperm concentration and sire. Theriogenology 2002; 57: 2105–2117 [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Igarashi H, Kawagoe J, Amita M, Hara S, Kurachi H. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol Reprod 2009; 80: 493–502 [DOI] [PubMed] [Google Scholar]
- 13.Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod 2001; 16: 909–917 [DOI] [PubMed] [Google Scholar]
- 14.Zhang N, Wakai T, Fissore RA. Caffeine alleviates the deterioration of Ca(2+) release mechanisms and fragmentation of in vitro-aged mouse eggs. Mol Reprod Dev 2011; 78: 684–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi MM, Manchester JK, Yang VC, Curato AD, Strickler RC, Lowry OH. Contrast in levels of metabolic enzymes in human and mouse ova. Biol Reprod 1988; 39: 295–307 [DOI] [PubMed] [Google Scholar]
- 16.Morado SA, Cetica PD, Beconi MT, Dalvit GC. Reactive oxygen species in bovine oocyte maturation in vitro. Reprod Fertil Dev 2009; 21: 608–614 [DOI] [PubMed] [Google Scholar]
- 17.Nabenishi H, Takagi S, Kamata H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. The role of mitochondrial transition pores on bovine oocyte competence after heat stress, as determined by effects of cyclosporin A. Mol Reprod Dev 2012; 79: 31–40 [DOI] [PubMed] [Google Scholar]
- 18.Tarazona AM, Rodríguez JI, Restrepo LF, Olivera-Angel M. Mitochondrial activity, distribution and segregation in bovine oocytes and in embryos produced in vitro. Reprod Domest Anim 2006; 41: 5–11 [DOI] [PubMed] [Google Scholar]
- 19.Nagano M, Katagiri S, Takahashi Y. ATP content and maturational/developmental ability of bovine oocytes with various cytoplasmic morphologies. Zygote 2006; 14: 299–304 [DOI] [PubMed] [Google Scholar]
- 20.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Gonçalves PB, Wolf E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod 2001; 64: 904–909 [DOI] [PubMed] [Google Scholar]
- 21.Tamassia M, Nuttinck F, May-Panloup P, Reynier P, Heyman Y, Charpigny G, Stojkovic M, Hiendleder S, Renard JP, Chastant-Maillard S. In vitro embryo production efficiency in cattle and its association with oocyte adenosine triphosphate content, quantity of mitochondrial DNA, and mitochondrial DNA haplogroup. Biol Reprod 2004; 71: 697–704 [DOI] [PubMed] [Google Scholar]
- 22.Merton JS, de Roos APW, Koenen EPC, Roelen BAJ, Vos PL, Mullaart E, Knijn HM. Bovine OPU-derived oocytes can be matured in vitro for 16–28 h with similar developmental capacity. Reprod Domest Anim 2012; 47: 1037–1042 [DOI] [PubMed] [Google Scholar]
- 23.Sugimura S, Matoba S, Hashiyada Y, Aikawa Y, Ohtake M, Matsuda H, Kobayashi S, Konishi K, Imai K. Oxidative phosphorylation-linked respiration in individual bovine oocytes. J Reprod Dev 2012; 58: 636–641 [DOI] [PubMed] [Google Scholar]
- 24.Somfai T, Kikuchi K, Kaneda M, Akagi S, Watanabe S, Mizutani E, Haraguchi S, Dang-Nguyen TQ, Inaba Y, Geshi M, Nagai T. Cytoskeletal abnormalities in relation with meiotic competence and ageing in porcine and bovine oocytes during in vitro maturation. Anat Histol Embryol 2011; 40: 335–344 [DOI] [PubMed] [Google Scholar]
- 25.Tian XC, Lonergan P, Jeong BS, Evans AC, Yang X. Association of MPF, MAPK, and nuclear progression dynamics during activation of young and aged bovine oocytes. Mol Reprod Dev 2002; 62: 132–138 [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y, First NL. In vitro fertilization of bovine oocytes in the presence of theophylline. Anim Reprod Sci 1993; 34: 1–18 [Google Scholar]
- 27.Takahashi Y, Hishinuma M, Matsui M, Tanaka H, Kanagawa H. Development of in vitro matured/fertilized bovine embryos in a chemically defined medium: influence of oxygen concentration in the gas atmosphere. J Vet Med Sci 1996; 58: 897–902 [DOI] [PubMed] [Google Scholar]
- 28.Nagano M, Katagiri S, Takahashi Y. Relationship between bovine oocyte morphology and in vitro developmental potential. Zygote 2006; 14: 53–61 [DOI] [PubMed] [Google Scholar]
- 29.Bavister BD, Leibfried ML, Lieberman G. Development of preimplantation embryos of the golden hamster in a defined culture medium. Biol Reprod 1983; 28: 235–247 [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y, Kanagawa H. Effect of oxygen concentration in the gas atmosphere during in vitro insemination of bovine oocytes on the subsequent embryonic development in vitro. J Vet Med Sci 1998; 60: 365–367 [DOI] [PubMed] [Google Scholar]
- 31.Takahashi Y, Kanagawa H. Effects of glutamine, glycine and taurine on the development of in vitro fertilized bovine zygotes in a chemically defined medium. J Vet Med Sci 1998; 60: 433–437 [DOI] [PubMed] [Google Scholar]
- 32.Takahashi Y, First NL. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 1992; 37: 963–978 [DOI] [PubMed] [Google Scholar]
- 33.Marei WF, Wathes DC, Fouladi-Nashta AA. Differential effects of linoleic and alpha-linolenic fatty acids on spatial and temporal mitochondrial distribution and activity in bovine oocytes. Reprod Fertil Dev 2012; 24: 679–690 [DOI] [PubMed] [Google Scholar]
- 34.Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development 1990; 109: 501–507 [DOI] [PubMed] [Google Scholar]
- 35.Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 1991; 30: 4480–4486 [DOI] [PubMed] [Google Scholar]
- 36.Rieger D. Batch analysis of the ATP content of bovine sperm, oocytes, and early embryos using a scintillation counter to measure the chemiluminescence produced by the luciferin-luciferase reaction. Anal Biochem 1997; 246: 67–70 [DOI] [PubMed] [Google Scholar]
- 37.Saeki K, Kato H, Hosoi Y, Miyake M, Utsumi K, Iritani A. Early morphological events of in vitro fertilized bovine oocytes with frozen-thawed spermatozoa. Theriogenology 1991; 35: 1051–1058 [DOI] [PubMed] [Google Scholar]
- 38.Thibault C, Gerard M, Menezo Y. Preovulatory and ovulatory mechanisms in oocyte maturation. J Reprod Fertil 1975; 45: 605–610 [DOI] [PubMed] [Google Scholar]
- 39.Suzuki H, Moriguchi M, Kida R, Moro Y. Delay in ovulation and fertilization and asynchronous pronuclear development in aged hamsters. J Reprod Dev 1996; 42: 15–22 [Google Scholar]
- 40.Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol Reprod 2013; 88: 67 [DOI] [PubMed] [Google Scholar]
- 41.Tang DW, Fang Y, Liu ZX, Wu Y, Wang XL, Zhao S, Han GC, Zeng SM. The disturbances of endoplasmic reticulum calcium homeostasis caused by increased intracellular reactive oxygen species contributes to fragmentation in aged porcine oocytes. Biol Reprod 2013; 89: 124 [DOI] [PubMed] [Google Scholar]
- 42.Hao ZD, Liu S, Wu Y, Wan PC, Cui MS, Chen H, Zeng SM. Abnormal changes in mitochondria, lipid droplets, ATP and glutathione content, and Ca(2+) release after electro-activation contribute to poor developmental competence of porcine oocyte during in vitro ageing. Reprod Fertil Dev 2009; 21: 323–332 [DOI] [PubMed] [Google Scholar]
- 43.Igarashi H, Takahashi T, Takahashi E, Tezuka N, Nakahara K, Takahashi K, Kurachi H. Aged mouse oocytes fail to readjust intracellular adenosine triphosphates at fertilization. Biol Reprod 2005; 72: 1256–1261 [DOI] [PubMed] [Google Scholar]
- 44.Van Blerkom J, Davis P. High-polarized (Delta Psi m(HIGH)) mitochondria are spatially polarized in human oocytes and early embryos in stable subplasmalemmal domains: developmental significance and the concept of vanguard mitochondria. Reprod Biomed Online 2006; 13: 246–254 [DOI] [PubMed] [Google Scholar]
- 45.Kruip TAM, Cran DG, van Beneden TH, Dieleman SJ. Structural changes in bovine oocytes during final maturation in vivo. Gamete Res 1983; 8: 29–47 [Google Scholar]
- 46.Van Blerkom J. Mitochondria as regulatory forces in oocytes, preimplantation embryos and stem cells. Reprod Biomed Online 2008; 16: 553–569 [DOI] [PubMed] [Google Scholar]