Abstract
Background
By 2011, South African prevention of mother-to-child transmission of HIV (PMTCT) programmes had reduced perinatal HIV transmission at 6-weeks of age to 2.7%. We investigated the profile of newly-diagnosed vertically-infected children and their mothers to identify short-falls in the PMTCT programme.
Methods
In this operational follow-up study, fieldworkers enrolled mothers of newly-diagnosed HIV-infected children up to 2 years of age at 5 major healthcare facilities in Johannesburg. Structured questionnaires and clinical record reviews were conducted and analysed to describe the population and assess factors associated with PMTCT uptake.
Results
289 mother-child pairs were enrolled. Timing of maternal HIV diagnosis influenced PMTCT access and feeding choices, and was associated with infants’ age at HIV diagnosis (7 weeks vs. 11 weeks vs. 31 weeks where mothers tested before, during or after the pregnancy respectively; p <0.0001). Women diagnosed before pregnancy (12%) were older (median 31 years) than those diagnosed during the index pregnancy (53% - median 27 years). Women diagnosed after delivery (35%) were younger (median 25 years, p<0.0001), of lower parity, and less likely to be South African citizens. In 81 cases (29%) late maternal diagnosis precluded any PMTCT access. Where women were diagnosed during or before pregnancy, the recommended PMTCT guidelines for mother and infant were followed in 86 (61%) pairs.
Conclusion
Failure to diagnose maternal HIV infection before delivery was the main reason for missing PMTCT prophylaxis and early infant testing. Timely maternal diagnosis enables PMTCT uptake, but implementation and follow-up gaps require attention to improve infant outcomes.
Keywords: HIV infection, pregnancy, vertical transmission, children, maternal diagnosis
INTRODUCTION
The antenatal HIV prevalence in South Africa was estimated at 29.5% in 2011 with 260,280 pregnant women requiring treatment for prevention of mother-to-child transmission (PMTCT) of HIV in 2010.1,2 The South African PMTCT guidelines were amended in 2010 aligning them with WHO recommendations,3,4 and the programme is reported to have 98.8% coverage nationally for antenatal HIV testing, 78.3% CD4 cell count testing, 91.8% antiretroviral (ARV) prophylaxis to mother and/or baby and feeding advice in 89%.5,6 The high coverage and improved efficacy of the interventions has resulted in a substantial reduction in new paediatric HIV infections at 6-weeks of age from 5.8% in 2009 to 3.5% in 2010 and 2.7% in 2011,6,7 however, this still translates into thousands of infants infected annually.1,8 Understanding the short-comings of PMTCT at the operational, community and individual level will help to direct future policy decisions. By describing the timing of HIV diagnosis and profile of PMTCT uptake in women with newly-diagnosed HIV-infected children under 2 years of age, we aimed to determine reasons for vertical HIV transmission and identify modifiable factors.
METHODS
The Finding Infants with HIV Disease: Evaluation of Resistance (FInHDER) study was a prospective observational study conducted between January and December 2011 at 3 hospitals and 2 clinics in Johannesburg, South Africa. Newly-diagnosed HIV-infected children (≤ 2 years of age) and their caregivers were recruited. Children were antiretroviral triple therapy (ART)-naïve or had received less than 2 weeks of ART.
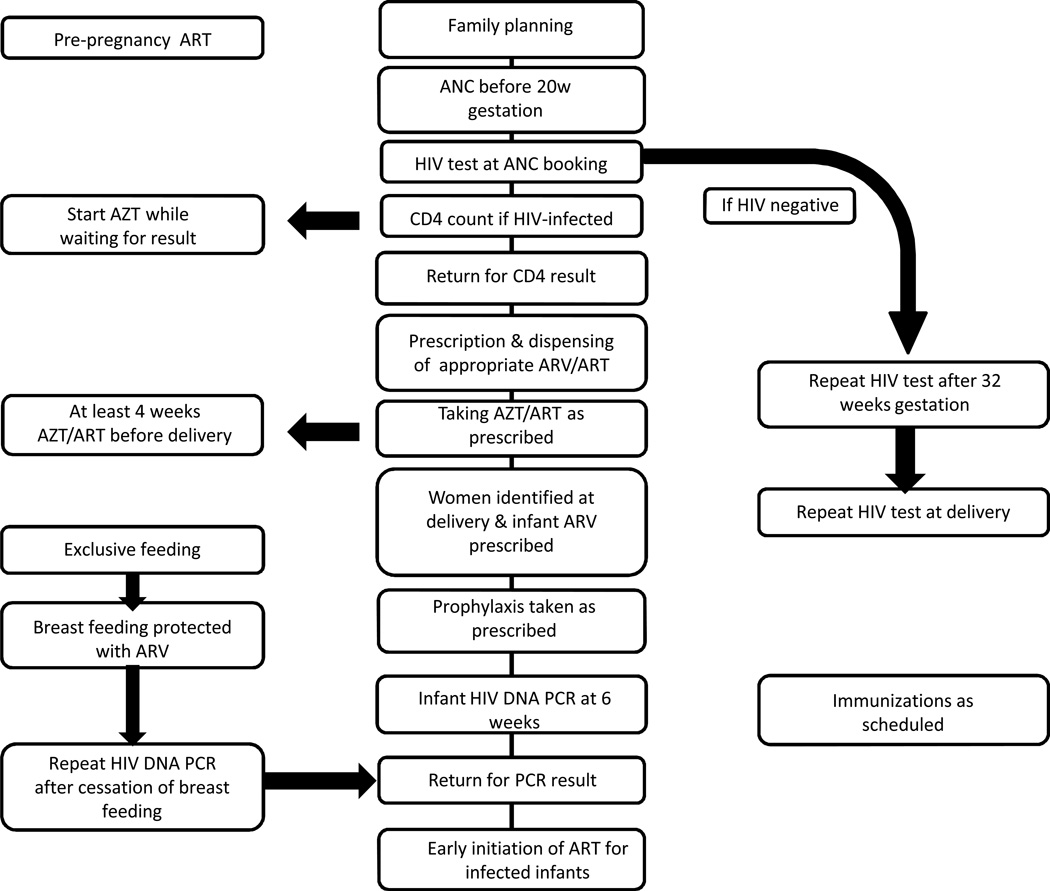
The South African PMTCT guidelines were updated in 2010 and the study sample captured children born before and after this change.9,10 After April 2010 (Figure 1), pregnant women were offered a single rapid HIV antibody assay at their first antenatal care (ANC) visit. If negative, a second test was offered after 32 weeks gestation. Positive results were confirmed (second rapid test) and CD4 cell count dictated further management. Women with CD4≤350 cells/mm3 were initiated on life-long ART. Women with CD4>350 cells/mm3 received twice daily zidovudine (AZT) from 14 weeks gestation, with single-dose nevirapine (sd-NVP), 3-hourly AZT and single-dose emtricitabine/tenofovir during labour. HIV-exposed infants received daily-dose nevirapine (dd-NVP) for 6 weeks. Dd-NVP was continued throughout breastfeeding if mothers were not on ART. HIV-exposed infants had an HIV DNA PCR test at their 6-week immunization visit. Before 2010, AZT was offered to women from 28 weeks gestation with sd-NVP during labour. Life-long ART was limited to those with CD4≤200 cells/mm3. Infants received sd-NVP at birth and either 7 or 28 days of AZT depending whether the mother had received prophylaxis for 4 weeks or less.9
Figure 1.
PMTCT Cascade (adapted from South African National PMTCT Guidelines 2010)10
Fieldworkers administered a structured questionnaire assessing obstetric history; antenatal care; timing of maternal diagnosis and access to PMTCT; and perceptions of the healthcare services. Economic factors included employment, residence and amenities (electricity, water and sanitation). Maternal psychological distress was measured according to the Kessler-10 system.11 Additional data were collected from medical records. A detailed review of each case identified psychosocial, healthcare system and biological factors that may have been implicated in transmission. The appropriateness of treatment was judged against the guidelines applicable during the pregnancy (2008 or 2010) using the recalled CD4 cell count from pregnancy or, if unavailable, the lowest CD4 cell count found on record in each case.
A sample size of 300 was calculated to address the expected prevalence of drug resistance (a separate aim of the study reported elsewhere).12 Data were collected on paper forms, entered into Microsoft Access and analyzed using SAS (Version 9.3, SAS Institute Inc., NC, USA). Categorical and continuous variables were compared using Chi-squared or Fisher Exact tests, and t-tests or Kruskal-Wallis methods respectively. Breastfeeding duration was assessed by Kaplan-Meier analysis. The study was approved by the Human Research Ethics Committee of the University of the Witwatersrand (M101148) and the Columbia University Institutional Review Board. All caregivers provided written informed consent.
RESULTS
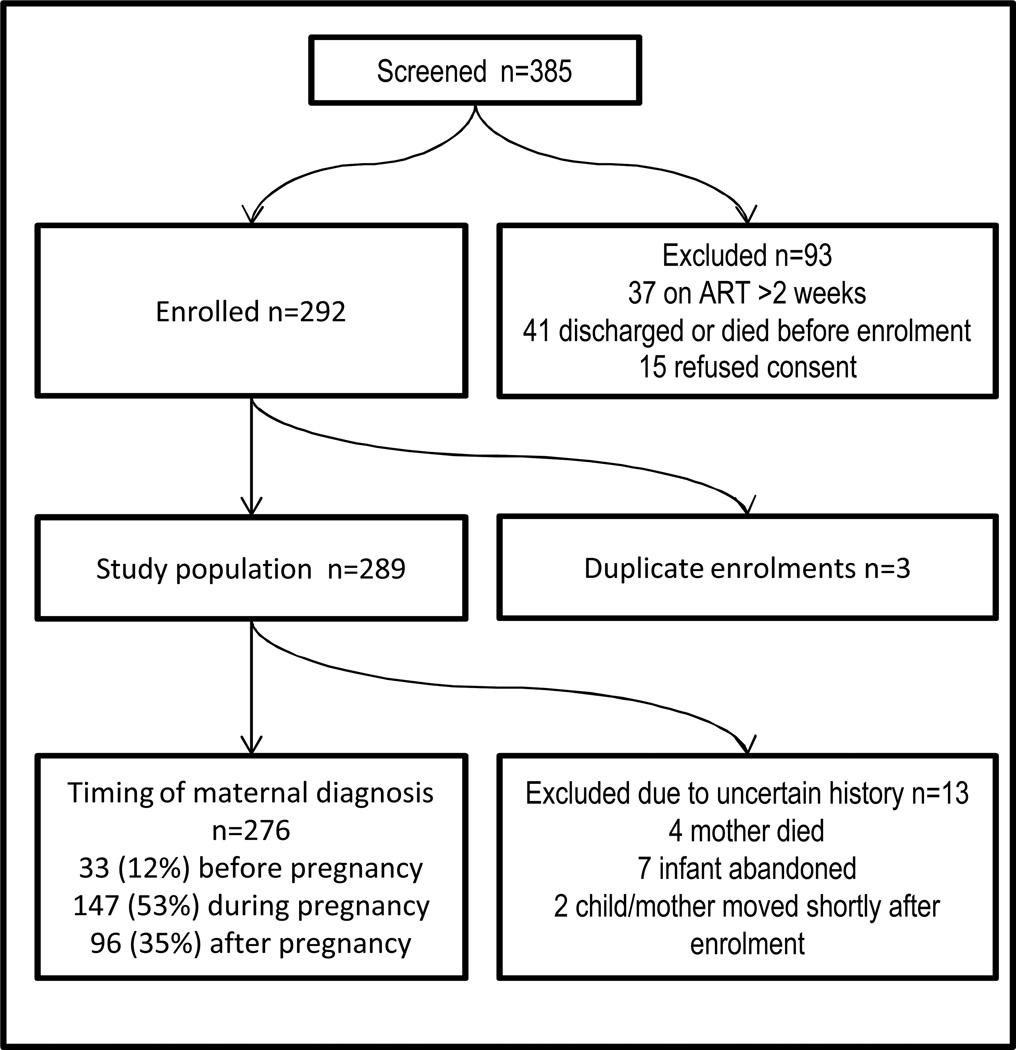
Between January and December 2011, 385 children were screened and 289 (75%) enrolled (Figure 2). Of those excluded: 15 refused consent, 37 had started ART more than 2 weeks prior, and 41 were discharged or died before enrolment. The 5 study sites contributed 1443 positive HIV PCR results within the district in 2011(PCR reports, National Health Laboratory Service); the study sample comprised 18% of all positive PCR results in the public sector for the City of Johannesburg over the study period.
Figure 2.
Study Population
Timing of maternal HIV diagnosis, i.e. (1) before, (2) during or (3) after the index pregnancy, was determined in 276 mother-child pairs who were categorized accordingly. Thirty-three women (12%) were diagnosed with HIV before, 147 (53%) during and 96 (35%) after the index pregnancy. Only 1 had been diagnosed with HIV during a previous pregnancy. One hundred and eighty (65%) women were diagnosed with HIV before or during pregnancy and had the opportunity to receive PMTCT: 105 women (66%) and 139 (81%) infants received treatment as recommended by national guidelines. In 86 pairs (61%), both mother and infant received appropriate treatment (p=0.66 for diagnosis before or during pregnancy). Of the entire cohort 81 (29%) mother/child pairs received no PMTCT intervention due to a maternal diagnosis only made postnatally.
The file review identified 256 (93%) subjects with at least one psychosocial, healthcare system or biological risk factor for vertical transmission (Table 3). In 162 (59%) cases maternal and healthcare system factors co-existed. There were no significant differences based on timing of maternal diagnosis.
Maternal characteristics (Table 1)
There were no differences in socioeconomic status or maternal stress levels between the 3 groups. Access to healthcare was also similar. Most women were in a relationship with the child’s father and had been for the preceding 2 years.
Women diagnosed before the index pregnancy were older (p<0.0001); of higher parity (p=0.013); and more likely to report the death of a previous child (p=0.012) than women diagnosed during or after the index pregnancy. They were also more likely to report the HIV-related death of a partner or other relative (35% vs. 20% vs. 17%; p=0.082). All had disclosed their HIV status to someone (partner or friends and family). In comparison, 17 (12%) women diagnosed during pregnancy and 17 (19%) diagnosed postpartum had not disclosed (p=0.003).
Antenatal Care and PMTCT (Table 2)
Most women attended at least one antenatal visit (n=259; 96%) at which 222 (92%) with unknown status had an HIV test. The median gestation at first ANC visit was 24 weeks (IQR: 16–28); a median of 4 visits (IQR: 3–6) were attended; and 244 (96%) attended on 2 or more occasions. Birth outcomes (birth weight, premature birth) were not different between groups. Overall, 183 women (66%) attended ANC after the PMTCT guideline change in 2010.
Women diagnosed before the index pregnancy (n=33) attended ANC before 20weeks gestation more commonly (p = 0.028) and most received some form of maternal and/or infant prophylaxis (p < 0.0001 compared with women diagnosed after the index pregnancy.) CD4 cell counts were assessed in 25 (89%) women at a median gestation of 21 weeks (IQR 13–24). Twenty women (61%) qualified for life-long ART although only 11 received it, and there was virological evidence of treatment failure in 10 of these. Of the 9 women who should have received ART, 3 reported not being offered ART, 5 reported they had not been emotionally ready to start treatment, and 1 delivered at 36 weeks gestation before starting treatment.
Women in this group reported a marginally higher incidence of perinatal complications, e.g. preterm or prolonged labour, premature rupture of membranes, umbilical cord prolapse, abnormal presentation or bleeding (p=0.056).
All 147 women diagnosed with HIV during the index pregnancy attended ANC, most (76%) after 20 weeks gestation. Ninety-one percent received some form of PMTCT (NVP, AZT or ART); however the applicable guidelines (2008 or 2010) were followed in only 86 women (66%). One hundred and thirty-one (89%) had a CD4 cell count during pregnancy of whom 128 (98%) attended ANC at least twice (and should have received treatment according to the result.) Twenty-four women (18%) initiated ART during pregnancy (median duration of treatment 11 weeks before delivery [IQR: 6–14]). Twenty-one (31%) women who qualified for ART according to CD4 cell count criteria received only AZT. Forty-seven women with CD4 cell counts above the threshold received AZT (appropriate treatment); however, by the time of study entry this had dropped below the threshold in 12 (26%). Nine women received only sd-NVP. A further 13 women took no PMTCT, the reasons stated: ART not dispensed (n=6), limited understanding of PMTCT and non-adherence (n=2), non-acceptance of HIV diagnosis (n=2), delivered outside South Africa (n=1), delayed diagnosis due to discrepant results (n=1), and conflict with husband regarding medication (n=1).
Women diagnosed with HIV after the index pregnancy (n=96) were diagnosed at a median of 6 months post-partum (IQR: 3–15 months) and had attended at least 1 (n=58; 60%), 2 (n=50; 52%) or 3 (n=44; 46%) immunization visits before infant HIV testing.
Twenty women diagnosed with HIV after delivery did not get tested antenatally. Nine did not attend ANC, mostly for reasons unknown, 2 reported being unaware of the pregnancy; 1 felt unprepared for motherhood and 1 feared dismissal from work. Eleven women booked at ANC (median 24 weeks gestation [IQR 20–28]) but did not test: 5 were not offered an HIV test, 3 did not receive HIV test results, and 3 refused an HIV test.
Seventy-three women (87% of those attending ANC in this group) reported a negative antenatal HIV test. Apart from younger age there were no other differences when compared with women who tested positive antenatally (Table 1). One woman insisted that she was only diagnosed postpartum although antenatal maternal prophylaxis was documented as having been dispensed. Fifteen infants received NVP prophylaxis, 10 born to women who learned their status early postpartum. In 4 instances infant NVP was documented as being issued although their mothers denied receipt of the ANC HIV test result. One infant started NVP at 1 month of age after maternal diagnosis.
Feeding choice and Infant Prophylaxis
Timing of maternal HIV diagnosis had a significant impact on feeding choice. Women diagnosed before the index pregnancy were less likely to breastfeed than women diagnosed during or after the index pregnancy (p<0.0001) even if they had breastfed a previous child. Of those who initiated breastfeeding, 27% (women diagnosed before) continued until 6 months (vs. 45% [women diagnosed during] vs. 56% [women diagnosed after pregnancy]; p=0.040). Approximately one third (n=48, 36%) of women who breastfed reported mixed feeding which did not differ between the groups.
There were 68 breastfed infants whose mothers were known to be HIV-infected before or within 3 days of the infant’s birth. Eleven (16%) received no prophylaxis postpartum (although 1 mother was on ART). Of the 57 (84%) infants who received any prophylaxis, 4 incorrectly received AZT and sd-NVP instead of dd-NVP after the 2010 change; 4 received only sd-NVP; and 44 received dd-NVP. Nine of these 44 babies (20%) received dd-NVP throughout breastfeeding and in an additional 4 pairs the mother was on ART. Thus only 13 of 53 breastfed infants (25%) received optimal ART protection after the 2010 guideline change.
Maternal and Infant outcome
Infants of mothers diagnosed prior to pregnancy were tested earlier compared to infants in the other 2 groups (p<0.0001). More than two-thirds of all children were unwell at diagnosis; the proportion being significantly higher (93%, p<0.0001) where maternal diagnosis occurred postpartum. In 59% of those diagnosed after the index pregnancy, the child’s clinical symptoms prompted the maternal HIV test. If postpartum maternal diagnosis is excluded, infants enrolled at the outpatient clinics were less likely to be ill at the time of diagnosis (2 [7%] vs. 185 [76%] at hospital, p<0.0001) and tested earlier than infants enrolled in the hospital (6 weeks vs. 14 weeks, p<0.0001). There was no difference between infants of mothers diagnosed before vs. during pregnancy stratified by enrolment site.
Eight women (3%) died. The timing of HIV diagnosis could be established in 4 cases: 2 were diagnosed before pregnancy (CD4 18 cells/mm3 in 1, unknown in the other); 1 during pregnancy (CD4 144 cells/mm3) and 1 after pregnancy (CD4 186 cells/mm3). Two had started ART. The causes of death were unknown.
DISCUSSION
While great improvements have been made in reducing mother-to-child HIV transmission in South Africa, the high HIV prevalence amongst pregnant women (29.5% in 2011)1 continues to result in perinatal infection. A combination of individual, biomedical and health system factors contribute to PMTCT failures.
Reported ANC attendance was high, but most women presented for their first visit after 14–16 weeks gestation, resulting in over 25% not attending the 4 ANC visits recommended by the WHO.13 Since early gestation at first presentation to ANC is the strongest predictor of ART at delivery, and time on ART is inversely related to risk of vertical transmission,14–16 this results in sub-optimal PMTCT. Depending on viral load, 12 to 16 weeks of maternal ART may be required to achieve viral suppression at delivery, longer than the median time on ART in our cohort.15,17,18 Late presentation for ANC in sub-Saharan Africa has been previously reported.14,19–21 Qualitative studies indicate that women (irrespective of HIV status) see little direct benefit of antenatal care unless they had experienced problems with the index or a previous pregnancy; often attending only to obtain the required documentation for delivery in a healthcare facility.19,20 Perceived poor quality of services, negative experiences with clinic staff, financial constraints and distance from the clinic have been reported as contributing factors.19, 21 Although not significant in our cohort (Table 1), these issues were reported by some individuals (Table 3) and further investigation is required.
Rates of antenatal HIV testing were high, consistent with a recent Medical Research Council PMTCT report that demonstrated an antenatal HIV testing rate of 98.8 % nationally.6,22 According to the report, while 91.8% of HIV-infected mothers and their infants received PMTCT prophylaxis, only 52% received the regimens recommended by the national guidelines (compared with 61% in our study). The reasons for this are difficult to elucidate: individual maternal factors (denial, lack of ‘readiness’) and “stock-outs” of drugs (isolated individual reports) may account for a portion of these failures in our cohort. Starting prophylaxis late, not starting ART according to guidelines (i.e. failure to initiate combination ART for women with low CD4 cell counts) and poor adherence to therapy were common factors. Simplifying the programme and reducing the number of steps could reduce errors as well as ensure optimal PMTCT.23 South Africa updated the PMTCT guidelines again in March 2013 offering combination ART to all HIV-positive pregnant women irrespective of the CD4 cell count.24 Our data indicate that personal circumstance affected participation in PMTCT and adherence to therapy. Further study is required to address how and why personal contexts dictate health-seeking behaviour and treatment adherence.
Rates of appropriate infant prophylaxis were high (81%) but only a quarter of breastfed infants received adequate protection for the duration of breastfeeding. Breastfeeding has been shown to confer a risk of approximately 1% per month in the absence of maternal ART or infant dd-NVP.25 This risk is higher among women with incident infections, a difficult group to reach being unaware of their HIV status. Mixed feeding is an additional risk for postnatal HIV transmission.26 In our study there was no association between awareness of HIV status and exclusive feeding practices and mixed feeding rates were higher than those reported in a recently published survey (36% v 18%).27 Women diagnosed before pregnancy were less likely to breastfeed and if they did, it was for a shorter duration. At the time, replacement feeding was supported by free infant formula, a service since phased out in favour of exclusive breastfeeding with ARV prophylaxis.28
Women aware of their HIV status before the index pregnancy were more likely to receive PMTCT and to test their infants early. This group was older, of higher parity and had booked earlier at ANC thereby receiving treatment (AZT or ART) for longer. Positive associations between adherence to PMTCT prophylaxis and older maternal age have been previously reported.29, 30 The death of a previous child may have influenced their health-seeking behaviour although it is unknown whether the deaths were HIV-related. Infants of the mothers in this group tested earlier (at 7 weeks old, close to the recommended 6 weeks) yet many (58%) were unwell at the time of diagnosis. This resonates with reports of high early mortality in perinataly infected infants (20% by 90 days).31 Despite early testing, morbidity in this group is high. In utero infection may be proportionately more common in the setting of good PMTCT coverage during late pregnancy and peripartum.32
Our data show that enrolment at the clinic rather than the hospital was associated with both earlier diagnosis and lower morbidity independent of the timing of maternal diagnosis, emphasising the importance of early infant diagnosis at the clinics. It is unclear why infant diagnosis occurred later when women were diagnosed with HIV during pregnancy (median. 11 weeks vs. 7 weeks if the maternal test was pre-conception). The reasons are probably multifactorial. One can speculate that women diagnosed before pregnancy were more willing to identify themselves as HIV-infected at infant clinics. Perhaps there was more acceptance of the diagnosis, disclosure of HIV status to family and friends may have played a role. PMTCT information, including need for PCR testing, is documented in the patient-held Road-to-Health booklet, though it is often incomplete and anecdotal reports suggest this is due to concern about inadvertent disclosure.
The majority of women diagnosed post-partum reported testing HIV negative during pregnancy. We relied on patient reporting of results (rapid tests are not recorded electronically) and feelings of guilt or denial surrounding HIV transmission may have influenced the responses. This could explain the discrepancies observed in women who reported testing HIV negative or not receiving an HIV result but for whom PMTCT-related medication is documented. Surveillance data in South Africa consistently demonstrates evidence of HIV exposure (as measured by the presence of serum antibodies to HIV) ranging from 4.1 to 6.9% in infants whose mothers reported a negative HIV test during pregnancy.6,33,34 Although blood-based rapid HIV tests have a high sensitivity, compliance with WHO standards and local quality assurance protocols cannot be guaranteed at each clinic and has resulted in field sensitivities as low as 87–95%.6,35,36 Despite these uncertainties, it is likely that many of the reported negative results represent newly- acquired infection during late pregnancy and breastfeeding. In Botswana, retesting negative women at delivery and at immunization visits identified an additional 1.3% and 2.4% of HIV-infected women respectively.37,38 In Johannesburg, testing at delivery found the incidence of HIV infection after a reported negative result to be 4.2%.39 Maternal acquisition of HIV in late pregnancy confers a high risk of vertical transmission due to the high viral loads during primary infection16,32,37,40–43 and postnatal acquisition of HIV was felt to account for up to 20% of breastfeeding-associated transmission in Zimbabwe.40 In the absence of a maternal diagnosis, infants cannot be identified as HIV-exposed and will not be captured by the HIV PCR testing programme. Repeat maternal testing is feasible, cost-effective and acceptable to women both at the time of delivery and at immunisation clinics.44,45 In South Africa most women deliver in healthcare facilities and 95% attend the 6-week immunisation visit.6,7,37–39,46,47 Repeat testing at these times has since been incorporated into updated PMTCT guidelines (April 2013).48 Postnatal maternal diagnosis had significant clinical implications and was often precipitated by the child’s hospitalisation. Delaying ART in HIV-infected infants results in significant morbidity and mortality.49–56
Limitations of our study include that not all infants screened were enrolled, some having already commenced ART or died. Our sample is not representative of every child diagnosed in the district during the study period. A control sample of HIV-exposed uninfected infants was not included for comparison. We relied on subject recall to establish the timing of maternal diagnosis and refusal of antenatal HIV testing, which is associated with high HIV prevalence,57 may have been under-reported. Lack of biological data meant that true incident infections after the first negative HIV test could not be confirmed. We did not have data on repeat antenatal HIV tests. Additionally, some subjects straddled the period of guideline change which affected our study in 2 ways: the 2010 guidelines offered a more effective protocol and confusion during the change-over may have resulted in missed opportunities.
We highlight important gaps in the operational implementation of PMTCT guidelines. Early identification of HIV-infected pregnant women (preferably pre-conception) remains central as late maternal diagnosis denies PMTCT to a significant number of infants. Once identified, all components of the PMTCT cascade need to be implemented. An attempt should be made to sensitize staff to the interplay of multiple sociodemographic and biological factors surrounding HIV infection and pregnancy. Retesting HIV-negative women during late pregnancy, at delivery, and postnatally will help to identify infants at high risk. Mothers should be encouraged to attend early infant diagnosis at six weeks or at any time before that if their infant is unwell. Public education about of the benefits of obstetric care and early ANC is vital while maternal feeding choices and exclusive feeding practices require support.
Supplementary Material
ACKNOWLEDGEMENTS
All participants are gratefully acknowledged. Many cases included stories of trauma yet also resilience. The study would not have been possible without the dedicated fieldworkers Phindile Moyo, Phumzile Nyamane, Jabulile Dlamini, Thuli Khumalo, Peace Khanyile and support staff Mercy Mia. The National Health Laboratory Service provided useful regular updates on PCR testing rates. The expanding PMTCT services are a result of tireless work of the South African Department of Health and its partners towards improved outcomes.
Funding
The U.S. President's Emergency Plan for AIDS Relief provided funding for this work (through the Eunice Kennedy Shriver National Institute of Child Health and Human Development - Supplement to HD 61255 Treatment Options for Protease-Inhibitor Exposed Children). The reported clinical services that participants accessed were part of the South African government healthcare provision. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Meetings where the work was presented:
Poster at the Southern African HIV Clinicians Society Conference, 25th–28th November 2013, Cape Town, South Africa.
The authors have no conflict of interest to disclose.
REFERENCES
- 1.National Department of Health. The National Antenatal Sentinel HIV and Syphilis Prevalence Survey. South Africa: National Department of Health; 2011. [Google Scholar]
- 2.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva: UNAIDS; 2010. [Google Scholar]
- 3.World Health Organization. PMTCT strategic vision 2010–2015: preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals: moving towards the elimination of paediatric HIV, December 2009. Geneva: World Health Organization; 2010. [Google Scholar]
- 4.World Health Organization. Rapid advice : use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants, version 2. Geneva: World Health Organization; 2009. Revised 2010. ed. [PubMed] [Google Scholar]
- 5.National strategic Plan 2007–2011. Mid Term Review 2010. South Africa: SANAC; 2009. [Google Scholar]
- 6.Goga AE, Dinh TH, Jackson DJ, for the SAPMTCTEstudy group . Evaluation of the Effectiveness of the National Prevention of Mother-to-Child Transmission (PMTCT) Programme Measured at Six Weeks Postpartum in South Africa, 2010. Medical Research Counsil, National Department of Health, PEPFAR, US Centres for Disease Control and Prevention; 2012. [Google Scholar]
- 7.Goga AE, Dinh TH, Jackson DJ, for theS APMTCTE study group Impact of the national prevention of mother-to-child transmission of HIV (PMTCT) program on perinatal mother-to-child transmission of HIV (MTCT) measured at six weeks postpartum, South Africa (SA); Washington. XIX International AIDS Conference.2012. [Google Scholar]
- 8.Mid-year Population Estimates 2011. South Africa: Statistics South Africa; 2011. [Google Scholar]
- 9.National Department of Health. Policy and Guidelines for the Implementation of the PMTCT Program. Pretoria: National Department of Health; 2008. 2008 ed. [Google Scholar]
- 10.National Department of Health. Clinical Guidelines: PMTCT (Prevention of Mother-to-Child Transmission) Pretoria: National Department of Health; 2010. 2010 ed. [Google Scholar]
- 11.Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–976. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn L, Hunt G, Technau K, et al. CROI. Atlanta, USA: 2013. Pre-treatment Drug Resistance Mutations among HIV+ Children <2 Years of Age Who Failed or Missed PMTCT: Johannesburg, South Africa. 2013 3rd to 6th March. [Google Scholar]
- 13.National Department of Health. Guidelines for Maternity Care in South Africa. South Africa: National Department of Health; 2007. [Google Scholar]
- 14.Stinson K, Boulle A, Coetzee D, Abrams EJ, Myer L. Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop Med Int Health. 2010;15:825–832. doi: 10.1111/j.1365-3156.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- 15.Myer L. Initiating antiretroviral therapy in pregnancy: the importance of timing. J Acquir Immune Defic Syndr. 2011;58:125–126. doi: 10.1097/QAI.0b013e31822ad573. [DOI] [PubMed] [Google Scholar]
- 16.Johnson LF, Stinson K, Newell ML, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e3182432f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chibwesha CJ, Giganti MJ, Putta N, et al. Optimal time on HAART for prevention of mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;58:224–228. doi: 10.1097/QAI.0b013e318229147e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Read PJ, Mandalia S, Khan P, et al. When should HAART be initiated in pregnancy to achieve an undetectable HIV viral load by delivery? AIDS. 2012;26:1095–1103. doi: 10.1097/QAD.0b013e3283536a6c. [DOI] [PubMed] [Google Scholar]
- 19.Myer L, Harrison A. Why do women seek antenatal care late? Perspectives from rural South Africa. J Midwifery Womens Health. 2003;48:268–272. doi: 10.1016/s1526-9523(02)00421-x. [DOI] [PubMed] [Google Scholar]
- 20.Simkhada B, Teijlingen ER, Porter M, Simkhada P. Factors affecting the utilization of antenatal care in developing countries: systematic review of the literature. J Adv Nurs. 2008;61:244–260. doi: 10.1111/j.1365-2648.2007.04532.x. [DOI] [PubMed] [Google Scholar]
- 21.Mathole T, Lindmark G, Majoko F, Ahlberg BM. A qualitative study of women's perspectives of antenatal care in a rural area of Zimbabwe. Midwifery. 2004;20:122–132. doi: 10.1016/j.midw.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Doherty T, Chopra M, Nsibande D, Mngoma D. Improving the coverage of the PMTCT programme through a participatory quality improvement intervention in South Africa. BMC public health. 2009;9:406. doi: 10.1186/1471-2458-9-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MH, Ahmed S, Preidis GA, et al. Low rates of mother-to-child HIV transmission in a routine programmatic setting in Lilongwe, Malawi. PloS one. 2013;8:e64979. doi: 10.1371/journal.pone.0064979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Department of Health SA. PMTCT Guidelines: revised March 2013. Pretoria: Department of Health, South Africa; 2013. In: Health, ed. [Google Scholar]
- 25.Bulterys M, Ellington S, Kourtis AP. HIV-1 and breastfeeding: biology of transmission and advances in prevention. Clin Perinatol. 2010;37:807–824. doi: 10.1016/j.clp.2010.08.001. ix-x. [DOI] [PubMed] [Google Scholar]
- 26.Iliff PJ, Piwoz EG, Tavengwa NV, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 27.Goga AE, Doherty T, Jackson DJ, et al. Infant feeding practices at routine PMTCT sites, South Africa: results of a prospective observational study amongst HIV exposed and unexposed infants - birth to 9 months. Int Breastfeed. 2012;7:4. doi: 10.1186/1746-4358-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Tshwane Declaration of support for breastfeeding in South Africa. S Afr J Clin Nutr. 2011;24:214. [Google Scholar]
- 29.Hassan AS, Sakwa EM, Nabwera HM, et al. Dynamics and constraints of early infant diagnosis of HIV infection in Rural Kenya. AIDS Behav. 2012;16:5–12. doi: 10.1007/s10461-010-9877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peltzer K, Mlambo G. Factors determining HIV viral testing of infants in the context of mother-to-child transmission. Acta Paediatr. 2010;99:590–596. doi: 10.1111/j.1651-2227.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- 31.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40:385–396. doi: 10.1093/ije/dyq255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lilian RR, Kalk E, Bhowan K, et al. Early Diagnosis of In Utero and Intrapartum HIV Infection in Infants Prior to 6 Weeks of Age. J Clin Microbiol. 2012;50:2373–2377. doi: 10.1128/JCM.00431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rollins N, Little K, Mzolo S, Horwood C, Newell ML. Surveillance of mother-to-child transmission prevention programmes at immunization clinics: the case for universal screening. AIDS. 2007;21:1341–1347. doi: 10.1097/QAD.0b013e32814db7d4. [DOI] [PubMed] [Google Scholar]
- 34.Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23:1255–1259. doi: 10.1097/QAD.0b013e32832a5934. [DOI] [PubMed] [Google Scholar]
- 35.Black V, von Mollendorf CE, Moyes JA, Scott LE, Puren A, Stevens WS. Poor sensitivity of field rapid HIV testing: implications for mother-to-child transmission programme. BJOG. 2009;116:1805–1808. doi: 10.1111/j.1471-0528.2009.02357.x. [DOI] [PubMed] [Google Scholar]
- 36.WHO prequalification of diagnostics, medicines and vaccines. 2010
- 37.Lu L, Motswere-Chirwa C, Legwaila K, et al. Abstract 15: HIV incidence in women during the first postpartum year: implications for PMTCT programs Francistown, Botswana, 2010. Rome, Italy: 3rd International Workshop on HIV Pediatrics; Reviews in Antiviral Therapy and Infectious Diseases 2011_8; 2011-17. [Google Scholar]
- 38.Lu L, Legwaila K, Motswere C, Smit M, W J, Creek T. Abstract 91: HIV incidence in pregnancy and the first post-partum year and implications for PMTCT programs, Francistown, Botswana, 2008; Montreal, Canada. 16th Conference on Retroviruses and Opportunistic Infections.2009. [Google Scholar]
- 39.Technau K. Johannesburg. South Africa: University of the Witwatersrand; 2009. Can a routine peri-partum HIV counselling and testing service for women improve access to HIV prevention, early testing and treatment of children? [Google Scholar]
- 40.Humphrey JH, Marinda E, Mutasa K, et al. Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. BMJ. 2010;341:c6580. doi: 10.1136/bmj.c6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magder LS, Mofenson L, Paul ME, et al. Risk factors for in utero and intrapartum transmission of HIV. J Acquir Immune Defic Syndr. 2005;38:87–95. doi: 10.1097/00126334-200501010-00016. [DOI] [PubMed] [Google Scholar]
- 42.Liang K, Gui X, Zhang YZ, Zhuang K, Meyers K, Ho DD. A case series of 104 women infected with HIV-1 via blood transfusion postnatally: high rate of HIV-1 transmission to infants through breast-feeding. J Infect Dis. 2009;200:682–686. doi: 10.1086/605123. [DOI] [PubMed] [Google Scholar]
- 43.Lilian RR, Kalk E, Technau KG, Sherman GG. Birth Diagnosis of HIV Infection on Infants to Reduce Infant Mortality and Monitor for Elimination of Mother-to-Child Transmission. Pediatr Infect Dis J. 2013 Apr 9; doi: 10.1097/INF.0b013e318290622e. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 44.Kim LH, Cohan DL, Sparks TN, Pilliod RA, Arinaitwe E, Caughey AB. The cost-effectiveness of repeat HIV testing during pregnancy in a resource-limited setting. J Acquir Immune Defic Syndr. 2013;63:195–200. doi: 10.1097/QAI.0b013e3182895565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chi BH, Bolton-Moore C, Holmes CB. Prevention of mother-to-child HIV transmission within the continuum of maternal, newborn, and child health services. Curr Opin HIV AIDS. 2013 Sep;8(5):497–502. doi: 10.1097/COH.0b013e3283637f7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Black S, Zulliger R, Marcus R, Myer L, Bekker L. Abstract P38: Acceptability and challenges of rapid antiretroviral initiation during pregnancy in Cape Town, South Africa; 1st Southern African HIV Clinicians' Society Conference; Cape Town, South Afrrica. 2012. 2012 25-28 November 2012. [Google Scholar]
- 47.Day C, Barron P, Massyn N, Padarath A, English R. District Health Barometer 2010/11. South Africa: Health Systems Trust; 2011. [Google Scholar]
- 48.National Department of Health. The South African Antiretroviral Treatment Guidelines 2013. Pretoria: National Department of Health; 2013. [Google Scholar]
- 49.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 51.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 52.Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS. 2009;23:101–106. doi: 10.1097/qad.0b013e32831c54bd. [DOI] [PubMed] [Google Scholar]
- 53.Dramowski A, Coovadia A, Meyers T, Goga A. Identifying missed opportunities for early intervention among HIV-infected paediatric admissions at Chris Hani Baragwanath Hospital, Soweto, South Africa. S Afr J HIV Med. 2011;12(4):16. [Google Scholar]
- 54.Leyenaar JK, Novosad PM, Ferrer KT, et al. Early clinical outcomes in children enrolled in human immunodeficiency virus infection care and treatment in lesotho. Pediatr Infect Dis J. 2010;29:340–345. doi: 10.1097/INF.0b013e3181bf8ecb. [DOI] [PubMed] [Google Scholar]
- 55.Obimbo EM, Mbori-Ngacha DA, Ochieng JO, et al. Predictors of early mortality in a cohort of human immunodeficiency virus type 1-infected african children. Pediatr Infect Dis J. 2004;23:536–543. doi: 10.1097/01.inf.0000129692.42964.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wamalwa D, Benki-Nugent S, Langat A, et al. Survival Benefit of Early Infant Antiretroviral Therapy is Compromised When Diagnosis is Delayed. Pediatr Infect Dis J. 2012;31:729–731. doi: 10.1097/INF.0b013e3182587796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mseleku M, Smith TH, Guidozzi F. HIV seropositive in pregnant South African women who initially refuse routine antenatal HIV screening. BJOG. 2005;112:370–371. doi: 10.1111/j.1471-0528.2004.00424.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.