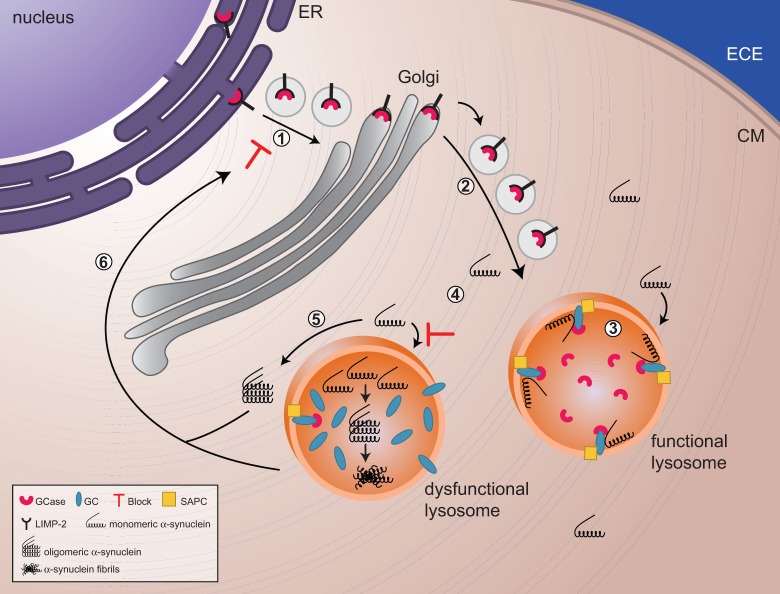
Figure 1.
The reciprocal relationship between glucocerebrosidase and α-synuclein. (1 and 2) Glucocerebrosidase (GCase) is sorted via the endoplasmic reticulum and Golgi to the lysosomes. (3) In lysosomes, glucocerebrosidase interacts with its substrate glucocerebroside (GC) as well as monomers of α-synuclein, facilitating the breakdown of both at acidic pH. (4) Decreased levels of glucocerebrosidase will result in a slowdown of α-synuclein degradation and a gradual build up of glucocerebroside substrate, with the eventual formation of α-synuclein oligomers and fibrils. (4) Impaired lysosomes, as a result of build up of substrate and/or α-synuclein oligomers and fibrils, will show impaired chaperone-mediated autophagy and autophagosome fusion, which implies that α-synuclein cannot undergo autophagy-mediated degradation, resulting in an increased accumulation of α-synuclein in the cytoplasm. (5) These soluble monomers will eventually assemble in oligomers and will block trafficking of glucocerebrosidase from the endoplasmic reticulum (ER) to the Golgi. ECE = extracellular environment; CM = Cell Membrane; SAPC = saposin C.