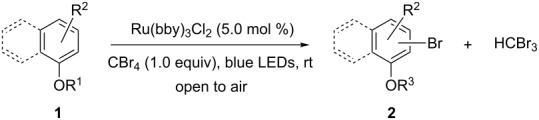
Table 2.
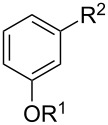
Scope of the photocatalytic bromination of phenols.
 | |||
| Entry | Substrate | Product | Yield (%)a |
 |
 |
Conditionsb | |
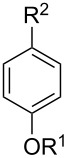
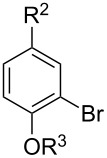
| 1 | R1 = TMS; R2 = OMe; R3 = H | 88 | |
| 2 | R1 = TMS; R2 = Me; R3 = H | 69 | |
| 3 | R1 = TMS; R2 = Cl; R3 = H | 58 | |
| 4 | R1 = TBS; R2 = OMe; R3 = H | 85 | |
| 5 | R1 = MOM; R2 = Me; R3 = H | 97 | |
 |
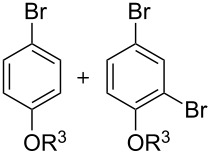 |
||
| 6 | R1 = TMS; R3 = H (3:2)c | 79 | |
| 7 | R1 = THP; R3 = H (3:2)c | 79 | |
 |
 |
||
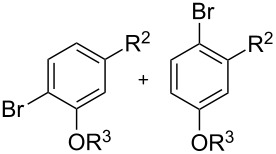
| 8 | R1 = TMS; R2 = OMe; R3 = H (2:1)c | 73 | |
| 9 | R1 = Ms; R2 = OMe; R3 = Ms (5:1)c | 95 | |
| 10 | R1 = Bn; R2 = OMe; R3 = Bn (5:3)c | 98 | |
| 11 | R1 = H; R2 = OMe; R3 = H (3:2)c | 40 | |
| 12 | 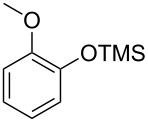 |
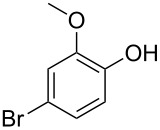 |
84 |
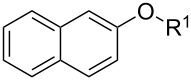 |
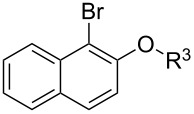 |
||
| 13 | R1 = TMS; R3 = H | 76 | |
| 14 | R1 = Me; R3 = Me | 98 | |
| 15 | 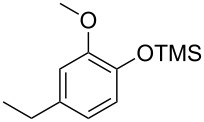 |
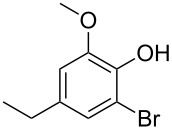 |
46 |
aIsolated yield based on complete consume of the starting material. bReaction conditions: substrate 1 (0.1 mmol), CBr4 (0.1 mmol), Ru(bpy)3Cl2 (5 mol %), dried CH3CN, blue LEDs (1 W), open to air. cRatio of the isomers in parentheses.
