FIGURE 6. Comparison of the longitudinal packing between PhuZ201-GDP, PhuZKZ-like-GDP and the three-stranded filament.
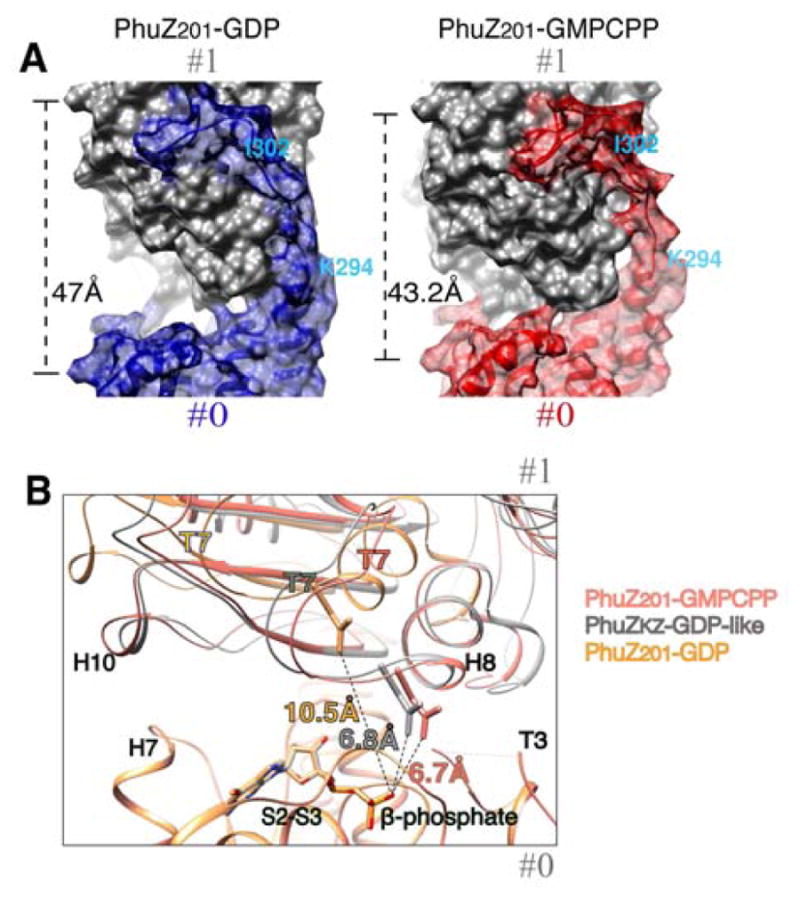
(A) and (B) Longitudinal subunits within a dimer are labeled as #0 and #1. (A) PhuZ201 dimers representative of the packing within the crystal (3r4v) (Kraemer et al., 2012) (left) vs the three-stranded filament (right) are shown as molecular surfaces. (left) PhuZ201 subunit packing within the crystal: the C-terminal tail of the subunit #0 in blue forms extensive interactions with the side of the subunit #1 in gray. The dimer has a relaxed longitudinal interface with 47 Å spacing. (right) PhuZ201 subunit packing within the three-stranded filament: the C-terminal tail of the subunit #0 in red forms weak longitudinal contacts with the side of the subunit #1 in gray. The dimer has a tense canonical tubulin/FtsZ longitudinal interface with 43.2 Å spacing. (B) Magnified view of the longitudinal interfaces in: PhuZ201-GMPCPP dimer in salmon, PhuZ201 mimicking subunit packing as in a PhuZKZ-GDP dimer (3ZBQ) (Aylett et al., 2013) in gray and a PhuZ201-GDP dimer (3r4v) (Kraemer et al., 2012) in yellow. The dimers were superimposed via the residues (2–271) corresponding to the N-terminal domains and the activation domains, but excluding the residues corresponding to the C-termini, of the subunits at minus ends. Measured distances from the catalytic Asp on the T7 loop to the β-phosphate are: 6.7 Å the three-stranded filament, 6.8 Å in PhuZKZ-GDP-like state, and 10.5 Å in PhuZ201-GDP. See also Movie S2.
