Abstract
During Drosophila metamorphosis, most larval cells die. Pupal and adult tissues form from imaginal cells, tissue-specific progenitors allocated in embryogenesis that remain quiescent during embryonic and larval life. Clonal analysis and fate mapping of single, identified cells shows that tracheal system remodeling at metamorphosis involves a classical imaginal cell population, and a population of differentiated, functional larval tracheal cells that re-enter the cell cycle and regain developmental potency. In late larvae, both populations are activated and proliferate, spread over and replace old branches, and diversify into various stalk and coiled tracheolar cells under FGF control. Thus, Drosophila pupal/adult tissue progenitors can arise both by early allocation of multipotent cells, and late return of differentiated cells to a multipotent state, even within a single tissue.
Drosophila larval tissues are composed of differentiated larval cells and imaginal cells. Imaginal cells are pupal and adult tissue progenitors that reside in clusters embedded in or attached to larval tissue (1). They remain quiescent during embryogenesis and part or all of larval life, then proliferate and differentiate into pupal and adult tissues at metamorphosis (2). By contrast, larval cells cease dividing and differentiate early in development; however, they typically enlarge and become polyploid during larval life (3). At metamorphosis, most larval cells die (4). Although some larval neurons (5) and muscles (6) are retained in adult tissues, no differentiated cells are known to re-enter the cell cycle and generate new cells and tissues. We show that tracheal (respiratory) system remodeling at metamorphosis is carried out by a classical imaginal cell population and another progenitor population that, like facultative stem cells in mammals (7), arises from differentiated cells.
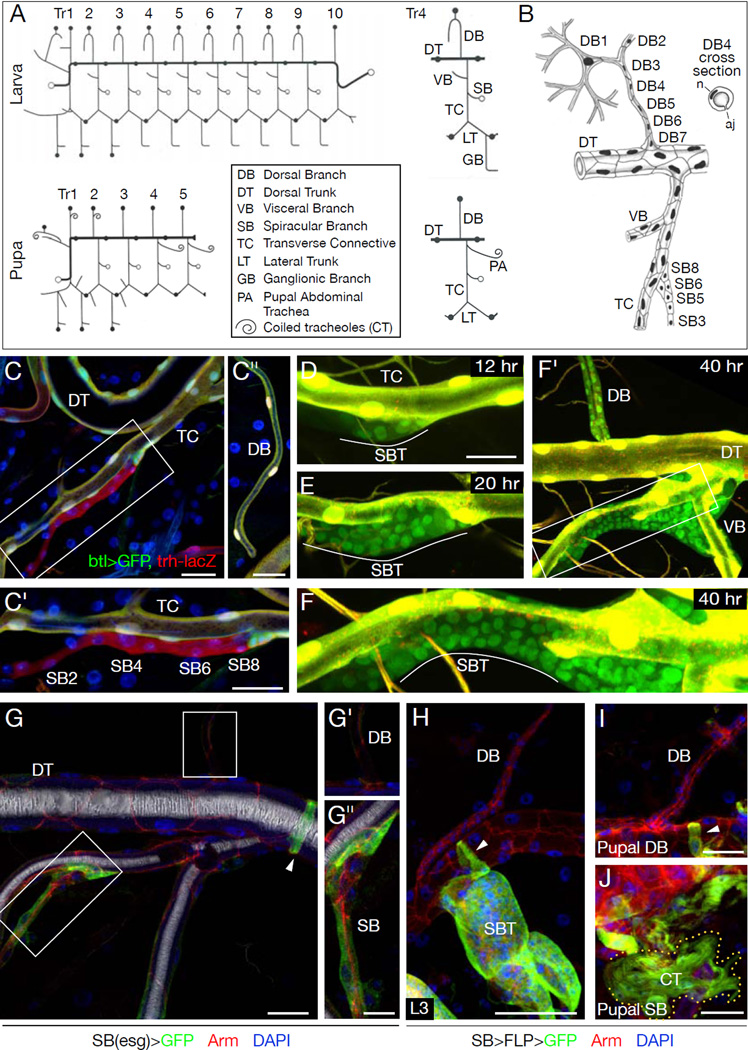
During embryogenesis, the tracheal system develops from segmentally-repeated groups of ~80 cells that express Trachealess transcription factor and invaginate, forming sacs attached to epidermis by a stalk of spiracular branch (SB) cells (8–10). Branches bud from the sacs and cells diversify primarily under control of Branchless FGF (Fibroblast Growth Factor), which activates Breathless FGFR (FGF Receptor) on tracheal cells (11, 12). At metamorphosis, posterior tracheal segments Tr6-Tr10 are lost (Fig. 1A); new branches form in Tr4 and Tr5 to supply posterior tissues, and in Tr2 to supply flight muscle (8, 13–16). Although most branches in Tr1-Tr5 are retained, most of their cells are replaced by imaginal cells (17, 18).
Figure 1. Spiracular branch tracheoblasts are not the source of dorsal branch tracheoblasts.
(A) Drosophila tracheal remodeling at metamorphosis. (Left) Schematics of larval and pupal tracheal system. Tr, tracheal metamere. Filled circles, positions of fusion cells connecting metameres. Open circles, attachments to epidermis. (Right) Branch names in Tr4. (B) Cellular structure of larval Tr4. DB contains terminal (DB1), fusion (DB2), and several stalk (DB3-7) cells. SB contains 6–8 cells (SB1-8). SBs are collapsed but open at molts for tracheal cuticle extrusion. n, nucleus; aj, autocellular junction. (C) SB in early L3 larva carrying trh-lacZ (anti-beta-galactosidase; red), btl>GFP (btl-Gal4; UAS-GFP; anti-GFP, green). Nuclei are DAPI-stained (blue). C', C": SB (boxed) and DB in same segment. All tracheal cells including SB and DB cells express trh-lacZ; all except SB cells express btl>GFP. (D–F) SB in "molecular timer" strain (btl-Gal4, UAS-GFP; UAS-DsRed) at indicated times in L3. (D) btl-Gal4 expression initiating in SB tracheoblasts (SBTs) visualized by UAS-GFP expression (green). DsRed (red) takes longer to mature so SBTs appear green, whereas tracheal cells in which btl-Gal4 was previously active express both proteins, so appear yellow. (E, F) Proliferating and spreading SBTs. (F') Low magnification of F. Fig. S5 shows low magnification of D, E. (G) Early L3 esg>GFP (esg-Gal4, UAS-GFP) larva immunostained for GFP (green) and Armadillo (cell junction marker; red). Blue, DAPI-stained nuclei. White (reflected light), air-filled lumen. Insets (G', G"), DB and SB (boxed). SB cells, but not DB or other tracheal cells except fusion cells (arrowhead), express esg. (H–J) Lineage tracing of SB (esg-expressing) cells with P127-Gal4; UAS-FLP; yAc-GFP. SBTs and fusion cells (arrowheads) express GFP lineage marker in late L3 (H) and pupae (I, J) where some SB tracheoblasts (e.g. PA) form CTs (outlined in J). DB tracheoblasts do not express lineage marker (H,I). Bars, 25 µm, except G',G", 10 µm.
Previous work indicated that imaginal tracheal cells (tracheoblasts) compose the SB (Fig. 1B). Unlike other tracheal cells, SB cells express imaginal marker escargot (esg), remain small and quiescent during embryonic and early larval life, and do not form gas transport tubes (Fig. 1C,G) (19) (8, 15, 17, 20, 21). BrdU incorporation studies showed that SB cells enter S phase at the beginning of the third larval period (L3) and divide 12–16 hours later (17). Proliferation continues for 24 hours, generating an expanding cluster of tracheoblasts at the SB-transverse connective (TC) junction (Fig. 1D,E). SB cells in the embryo and early larva express trachealess (trh), but, unlike most other tracheal cells (22), do not express the Trachealess target gene breathless (btl) (Fig. 1C): the tracheal program is apparently arrested at this step. When activated in L3, they turn down esg and turn on btl as they proliferate and leave the SB (Fig. 1D–F).
We developed a “molecular timer” strain (23) that highlights the burst of btl expression in activated tracheoblasts, which allowed us to distinguish them from the larval tracheal cells they migrate over and replace (Fig. 1D–F). SB tracheoblasts followed stereotyped paths. In Tr4, they migrated along the TC onto the visceral branch (Fig. 1 F'); later, some differentiated into coiled tracheolar (CT) cells (see Fig. 1J). Tracheoblasts respected specific boundaries, never spreading into neighboring tracheal segments or populating the dorsal trunk (DT; Fig. 1F'). However, tracheoblasts were observed on the other side of the DT, along the dorsal branch (DB) of Tr4 and other anterior DBs (Fig. 1F') (17).
If DB tracheoblasts arise from SB tracheoblasts, they would have to move across the DT to reach the DB. However, tracheoblasts were never seen crossing the DT. To exclude this possibility, the fate of SB tracheoblasts was mapped using SB-specific FLP recombinase to permanently label SB cells and their descendants (Fig. 1G–J). This labeled all tracheoblasts migrating out of the SB (Fig. 1H,J), but not DB tracheoblasts (Fig. 1H,I). Hence, DB tracheoblasts arise independently.
To identify the source of DB tracheoblasts, we scrutinized early L3 larval DBs, which comprise 5–7 cells (Fig. 1B) (21), but found no additional cells or cells with the distinctive small size and nuclear morphology of SB tracheoblasts. The positions and number of tracheoblast clones in a clonal analysis of larval DBs (Fig. S1, SOM text) suggested DB tracheoblasts arise from ~4–5 progenitors along the DB.
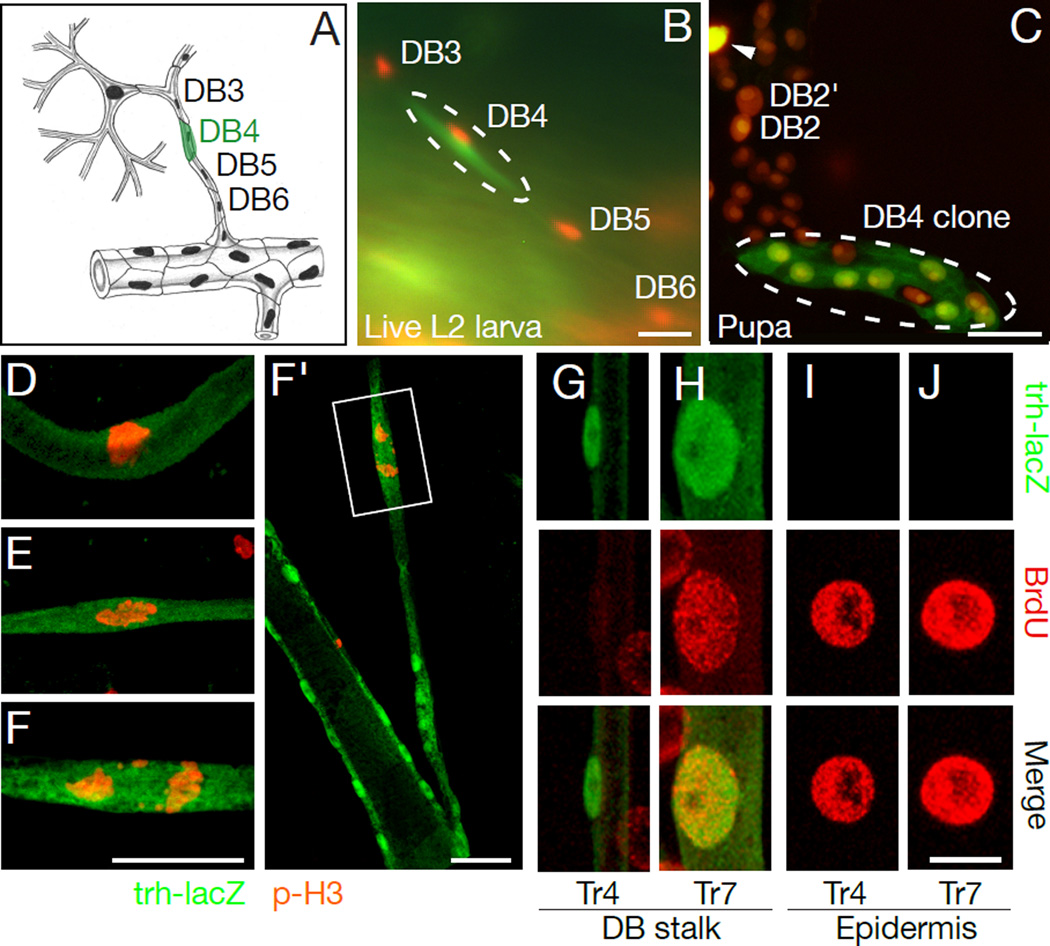
We considered whether differentiated stalk cells (DB3-DB7) might be the source of DB tracheoblasts (15, 17). To test this, we used a heat-inducible FLP transgene to permanently label and trace the fate of individual tracheal cells identified in live L2 larvae (Fig. 2A–C; Tables 1, S1). This demonstrated that larval DB stalk cells are the source. Individual stalk cells displayed a range of proliferative capacities, giving rise to 2–22 tracheoblasts (8.3±7.0, mean±S.D.), although occasionally a labeled stalk cell failed to proliferate or degenerated, the standard fate of DB1 and DB2 cells (Table 1, Fig. S2).
Figure 2. DB stalk cells proliferate and differentiate into tracheoblasts.
(A–C) Fate mapping a DB4 stalk cell (clone O, Table I). (A) Schematic showing labeled cell. (B) DB4 cell labeled with cytoplasmic GFP. All tracheal nuclei express DsRed (btl-Gal4; UAS-nuclearDsRed). (C) Same DB three days later. Labeled DB4 generated 7-cell clone. DB2 and contralateral DB2 (DB2') are indicated along with degenerating contralateral DB1 (arrowhead), which was also labeled in L2 larva. (D–F) DB4 cells at three different stages of mitosis in L3 larvae immunostained for phospho-histone H3 (red) and beta-galactosidase (trh-lacZ, green). Tubular DB4 cell divides along short axis of tube (F, F’). Fig. S6 shows low magnification. (G–J) Individual DB stalk cells in Tr4 and Tr7, and nearby epidermal cells of newly molted trh-lacZ L3 larva labeled with BrdU during L1 and L2 then co-stained after molting for BrdU (red) and beta-galactosidase (green). Fig. S7 shows low magnification. Bars, 25 µm, except G-J,10 µm.
Table I.
Fate mapping of single larval Dorsal Branch cells
| Clone | Marked cell | No. cells in clone |
Clone cell fates |
Class+ | |||||
|---|---|---|---|---|---|---|---|---|---|
| US^ | MS^ | CT^ | Dead | Lost | |||||
| A | DB1 | Tr5 | 1† | 0 | † | † | 1 | 0 | 0 |
| E | DB1 | Tr2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| G | DB2 | Tr2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| H | DB3 | Tr3 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| I | DB3 | Tr2 | 2 | 2 | 0 | 0 | 0 | 0 | I |
| J | DB3 | Tr2 | 5 | 5 | 0 | 0 | 0 | 0 | I |
| K | DB3 | Tr2 | 8 | 3 | 1 | 4 | 0 | 0 | II |
| L | DB3 | Tr2 | 12 | 7 | 1 | 4 | 0 | 0 | II |
| M | DB3 | Tr2 | 21 | 18 | 2 | 1 | 0 | 0 | II |
| N | DB4 | Tr3 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| O | DB4 | Tr2 | 7 | 7 | 0 | 0 | 0 | 0 | I |
| P | DB5 | Tr2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Q | DB5 | Tr3 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| R | DB5 | Tr3 | 14† | 14 | † | † | 0 | 0 | I |
| S | DB5 | Tr2 | 14 | 6 | 2 | 6 | 0 | 0 | II |
| T | DB6 | Tr5 | 1† | 0 | † | † | 1 | 0 | 0 |
| U | DB6 | Tr3 | >6 | >6 | 0 | 0 | 0 | 0 | I |
| V | DB6 | Tr2 | 10 | 4 | 0 | 6 | 0 | 0 | II |
| W | DB7 | Tr3 | 14 | 14 | 0 | 0 | 0 | 0 | I |
US, unicellular stalk; MS, multicellular stalk; CT, coiled tracheolar cell
Class 0, no proliferation, followed by necrosis or death; Class I, proliferation generating cells of the same fate, Class II, proliferation generating cells of multiple fates
Clone analyzed at W3L, before cell differentiation.
DB stalk cells have a complex morphology unlike typical progenitor or stem cells: they are tubular, with autocellular junctions, some (DB3 cells) forming Y-shaped tubes (Fig. 1B). Yet these cells become proliferating, migrating DB tracheoblasts while maintaining contacts with neighboring tracheal cells (Fig. 2D–F).
Phosphohistone H3 staining (Fig. 2D–F) showed DB stalk cells in anterior segments begin dividing 14–16 hours after the second molt. Even before they divide (Fig. 2G), they have smaller nuclei than other larval tracheal cells, including DB stalk cells in posterior segments (Fig. 2H), which are otherwise indistinguishable but do not give rise to tracheoblasts. BrdU labeling of newly molted third instar larvae showed anterior DB stalk cells do not incorporate the label (Fig. 2G), implying they do not endoreplicate and presumably remain diploid, unlike posterior DB cells and other differentiated larval cells (Fig. 2H–J), most of which endoreplicate and become polyploid (3).
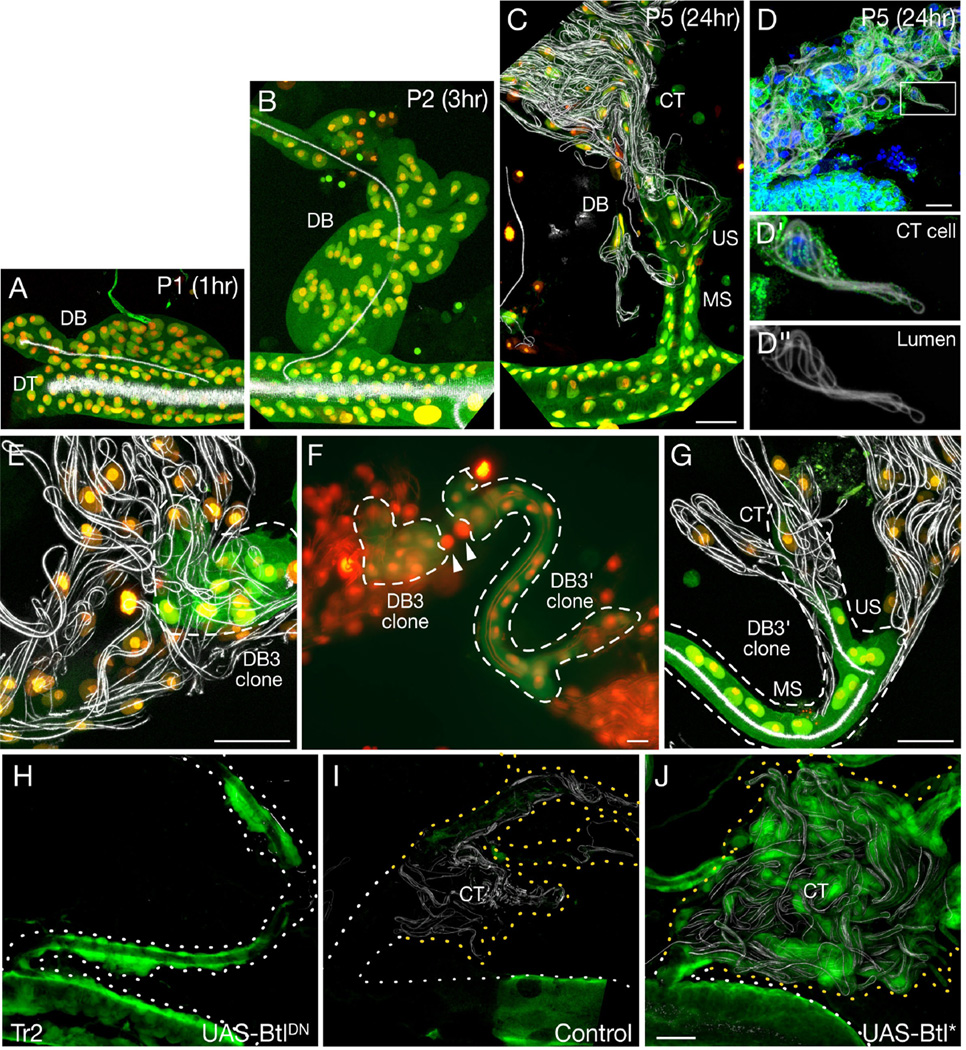
Although most DB tracheoblasts form multicellular stalks of pupal DBs (Table 1), in Tr2 they form more elaborate structures (Fig. 3A–D). After proliferating and spreading along the DB (Fig. 3A,B), they aggregate (Fig. 3B), form secondary branches (Fig. 3C), and differentiate into multicellular stalks (MS), unicellular stalks (US), and Blistered (DSRF)-expressing CT cells with coiled intracellular lumens (Figs. 3C,D, S3) that unfurl on flight muscle (8, 24). Fate mapping showed that single DB stalk cells in Tr2 routinely formed mixed clones containing MS, US, and CT cells (Fig. 3E–G, Tables 1, S1). Thus, DB stalk cells in Tr2 transform into multipotent tracheoblasts that can proliferate and acquire different fates.
Figure 3. DB stalk cells are multipotent progenitors.
(A–C) Tr2 DB tracheoblast morphogenesis in btl-Gal4; UAS-nDsRed; UAS-GFP pupae at pupal stages and times after pupariation indicated. Tracheal cytoplasm appears green (GFP), nuclei yellow (GFP, nDsRed) and air-filled lumen white (reflected light). MS, multi-cellular stalk; US, unicellular stalk; CT, coiled tracheoles. (D) CT cell cluster along Tr2 DB of btl-Gal4/+; UAS-Apc2-GFP/+ pupa showing microtubules (APC-GFP, green), nuclei (DAPI, blue) and air-filled lumen (reflected light, white). (D', D") Boxed CT cell with coiled lumen. (E–G) Fate mapping of L2 larva DB3 cell in Tr2 and contralateral DB3 (DB3') analyzed at stage P5 (F). DB3 generated 12-cell clone (E; clone L, Table 1); DB3' generated 21-cell clone (G; clone M). Both clones contain MS, US and CT cells. (H–J) Tr2 tracheal segment of btl-Gal4, UAS-GFP/tubulin Gal80TS; btl-Gal4, UAS-GFP/UAS-DNbtl (BtlDN) or UAS-, λbtl (Btl*) animals grown at 18°C and shifted to 30°C in late L2 to express dominant negative (H) or constitutively active Breathless (Btl*; J), or left at 18°C as control (I). Dotted lines, DBs and DTs; yellow dots, regions with CTs. Tracheal cells in experimental animals express GFP; expression in control (I) is inhibited by Gal80ts so tracheal cells were visualized by reflection (white) and DAPI staining. Bars, 25 µm.
Bnl/Btl signaling controls cell fate selection in the embryo (11, 12). To test for function in pupal tracheoblasts, we generated btl- clones. These rarely formed CT cells (Fig. S3, Table S2). Likewise, conditional expression of dominant negative Btl in the L3 tracheal system reduced or eliminated secondary branches and CT cells (Fig. 3H,I). Constitutively-active receptor induced ectopic secondary branches and CT cells throughout the tracheal system, including all anterior DBs (Figs. 3J, S4). Thus, DB tracheoblasts in anterior segments can acquire new tracheal fates, and FGF signaling also plays a critical role in reselecting cell fates when DB stalk cells are reactivated.
Anterior DB stalk cells are the first differentiated cells in Drosophila shown to reenter the cell cycle and regain developmental potency. They regain the same abilities to proliferate, spread, and differentiate into various tracheal cell types as SB tracheoblasts, classical imaginal cells that remain quiescent -- blocked in tracheal outgrowth and cell diversification -- during embryonic and most of larval life. This suggests that both types of tracheal progenitors arrive at a similar state, one by early developmental arrest (SB tracheoblasts), the other by late return to an earlier state (anterior DB stalk cells). The only known features that distinguish these cells from tracheal cells that lack progenitor potential (including posterior DB stalk cells that are otherwise indistinguishable from anterior DB stalk cells) are their small nuclear size and lack of endoreplication (Table S3). These features may be part of a program that maintains, or allows cells to regain, the proliferative and diversification potential of early tracheal cells. This program is operative in imaginal tracheal cells, and can apparently be implemented in other tracheal cells, independent of their differentiation program.
The blurring of the distinction between imaginal and differentiated larval cells in Drosophila parallels a current debate about adult stem cells in mammals (25). Some mammalian tissues have dedicated stem cells maintained in a primitive state (26). However, other tissues may rely on facultative stem cells, differentiated cells that re-enter the cell cycle to replenish lost cells (7). Our results show that progenitors with each of these features are present in a single Drosophila tissue and both play crucial roles. This provides a tractable system for dissection of the arrest of a tissue-specific developmental program and reversal to an earlier, more plastic state, important steps in tissue engineering, repair, and cancer.
Supplementary Material
References and Notes
- 1.Harbecke R, et al. Int J Dev Biol. 1996;40:197. [PubMed] [Google Scholar]
- 2.Kylsten P, Saint R. Dev Biol. 1997;192:509. doi: 10.1006/dbio.1997.8770. [DOI] [PubMed] [Google Scholar]
- 3.Edgar B, Orr-Weaver T. Cell. 2001;105:297. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 4.Yin V, Thummel C. Semin Cell Dev Biol. 2005;16:237. doi: 10.1016/j.semcdb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Williams D, Truman J. J Neurobiol. 2005;64:24. doi: 10.1002/neu.20151. [DOI] [PubMed] [Google Scholar]
- 6.Klapper R. Mech Dev. 2000;95:47. doi: 10.1016/s0925-4773(00)00328-2. [DOI] [PubMed] [Google Scholar]
- 7.Rawlins E, Hogan BLM. Development. 2006;133:2455. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 8.Manning G, Krasnow M. In: The Development of Drosophila melanogaster. Martinez A, Bate M, editors. I. Cold Spring Harbor, NY: CSHL Press; 1993. pp. 609–685. [Google Scholar]
- 9.Wilk R, Weizman I, Shilo B. Genes Dev. 1996;10:93. doi: 10.1101/gad.10.1.93. [DOI] [PubMed] [Google Scholar]
- 10.Isaac D, Andrew D. Genes Dev. 1996;10:103. doi: 10.1101/gad.10.1.103. [DOI] [PubMed] [Google Scholar]
- 11.Zelzer E, Shilo B. Bioessays. 2000;22:219. doi: 10.1002/(SICI)1521-1878(200003)22:3<219::AID-BIES3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Ghabrial A, Luschnig S, Metzstein M, Krasnow M. Annu Rev Cell Dev Biol. 2003;19:623. doi: 10.1146/annurev.cellbio.19.031403.160043. [DOI] [PubMed] [Google Scholar]
- 13.Pihan J. Arch. Zool. Exp. Gén. 1968;109:287. [Google Scholar]
- 14.Whitten J. In: The Genetics and Biology of Drosophila. Ashburner M, Wright T, editors. 2d. New York: Academic Press; 1980. pp. 499–540. [Google Scholar]
- 15.Sato M, Kornberg T. Dev Cell. 2002;3:195. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 16.Cabernard C, Affolter M. Dev Cell. 2005;9:831. doi: 10.1016/j.devcel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Guha A, Kornberg T. Dev Biol. 2005;287:192. doi: 10.1016/j.ydbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Kitada Y, Tabata T. Dev Biol. 2008;318:247. doi: 10.1016/j.ydbio.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Material and methods are available as supporting material on Science online.
- 20.Fuse N, Hirose S, Hayashi S. Development. 1996;122:1059. doi: 10.1242/dev.122.4.1059. [DOI] [PubMed] [Google Scholar]
- 21.Samakovlis C, et al. Development. 1996;122:1395. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- 22.Ohshiro T, Saigo K. Development. 1997;124:3975. doi: 10.1242/dev.124.20.3975. [DOI] [PubMed] [Google Scholar]
- 23.Ghabrial A, Krasnow M. Nature. 2006;441:746. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- 24.Shafiq S. Quarterly Journal of Microbiological Sciences. 1963;104:135. [Google Scholar]
- 25.Dor Y, Melton D. Cell Cycle. 2004;3:1104. [PubMed] [Google Scholar]
- 26.Weissman I, Anderson D, Gage F. Annu Rev Cell Dev Biol. 2001;17:387. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 27.We thank lab members for advice, reagents, and discussion. M.A.K. is an investigator of HHMI; M.W. was an HHMI fellow of the LSRF
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.