Figure 1. THC deregulating effect on microtubule dynamics in growth cones.
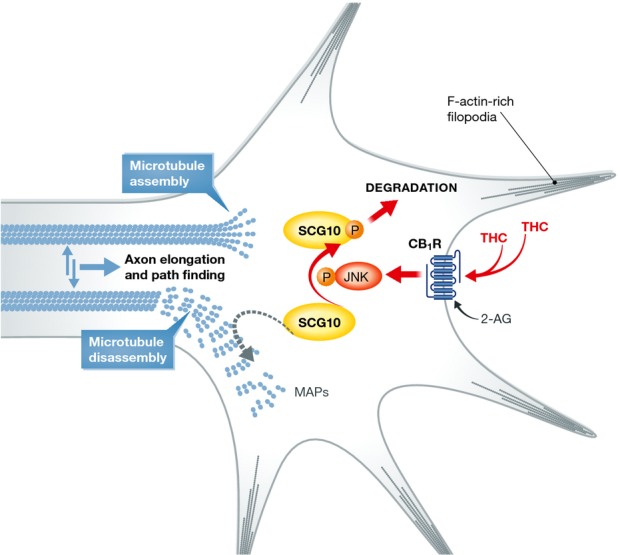
An integrated signaling transduced by CB1 via JNKs and executed by SCG10 is disrupted by THC during the corticogenesis of prenatal brains exposed to a dose of THC (3 mg/kg/day, embryonic day E5.5–17.5) non-aversive to dams. SCG10 is normally enriched near CB1 receptors in growth cone-like structures, mainly traversing the pallio-subpallial boundary, where its microtubule-destabilizing action represents an important factor for the dynamic assembly and disassembly of growth cone microtubules during axonal elongation. Phosphorylation at Ser62 and Ser73 by JNK1, the active brain-specific JNK isoform, exerted also through the tonic action of the endocannabinoid 2-AG, promotes SCG10 degradation with ensuing fine regulation of microtubule dynamics and axodendritic morphology. Chronic THC administration during cortical development disrupts this action through both reduced 2-AG biosynthesis and CB1 expression, and excessive SCG10 phosphorylation and degradation. THC also increases the formation of F-actin-rich filopodia in the distant motile axon segment. 2-AG, 2-arachidonoylglycerol; CB1R, cannabinoid receptor type 1; JNK1, c-Jun N-terminal kinase 1; MAPs, microtubule-associated proteins; THC, Δ9-tetrahydrocannabinol; SCG10, superior cervical ganglion 10.
