Abstract
Anti-smoking public service announcements (PSAs) often use persuasive arguments to attempt to influence attitudes about smoking. The persuasiveness of a PSA has previously been associated with factors that influence the cognitive processing of its message. Genetic factors that influence cognitive processing might thus affect individuals' responses to the persuasive arguments presented in PSAs. In the current study, we examined polymorphisms in the genes encoding brain-derived neurotrophic factor (BDNF Val66Met) and catechol-O-methyltransferase (COMT Val158Met), which affect cognitive processing in the prefrontal cortex, to identify genetic factors associated with self-reported outcomes of message processing, perceived effectiveness, and quitting intentions among smokers viewing PSAs. 120 smokers viewed sets of 4 PSAs which varied with respect to features of argument strength and message sensation value. We observed significant associations of BDNF genotype with central processing, narrative processing, perceived effectiveness of the anti-smoking PSAs, and participant quitting intentions; the BDNF Met allele was associated with lower scores on all these measures. Central processing acted as a mediator of the association of genotype with quitting intentions and perceived effectiveness. There was a significant interaction of COMT genotype by argument strength in the model of narrative processing, such that individuals homozygous for the COMT Val allele reported higher narrative processing in the high argument strength condition, but not in the low argument strength condition. To our knowledge, this is the first study to identify genetic factors associated with cognitive processing of anti-smoking public service announcements.
Keywords: BDNF, COMT, genetics, cognitive processing, public service announcements
Introduction
Tobacco smoking is the leading preventable cause of death worldwide (World Health Organization, 2009). Anti-smoking advertising campaigns using public service announcements (PSAs) have been employed in efforts to decrease smoking prevalence (Siegel, 1998; Biener, et al., 2000; Messer et al., 2007). Anti-smoking PSAs often use persuasive arguments to influence public attitudes about smoking, and features affecting cognitive processing of the arguments influence the perceived persuasiveness of PSAs (Biener et al., 2000; Leshner & Cheng, 2009; Strasser et al., 2009). Functional MRI has shown activation in regions of the prefrontal cortex (PFC) associated with memory encoding in response to effective persuasive arguments (Falk et al., 2010b). Further, activation in the PFC in response to persuasive messages predicts behavior change (Falk et al., 2010a). Indeed, accuracy of recall of anti-smoking PSAs correlates with PFC activation (Langleben et al., 2009), and increases in PFC activation while viewing anti-smoking PSAs predict quitting success (Chua et al., 2011). Therefore, factors influencing cognitive processing in the PFC might influence the effectiveness of anti-smoking PSAs.
Dopamine is one of the primary neurotransmitters in the PFC, and regulation of dopamine levels is a key component of optimizing PFC function (Cools & D'Esposito, 2011). Catechol-O-methyltransferase (COMT) degrades dopamine and is the primary regulator of dopamine levels in the PFC. A common polymorphism in the gene encoding COMT results in a valine to methionine substitution at codon 158 (Val158Met). The COMT Met allele is associated with reduced enzyme activity, leading to higher dopamine levels in the brain (Chen et al., 2004). COMT Met carriers outperform COMT Val homozygotes in tasks measuring working memory and PFC function (Egan et al., 2001; Goldberg et al., 2003). Furthermore, fMRI studies incorporating working memory tasks have shown greater increases in activation and less task-related deactivation in the PFC in COMT Val homozygotes at the same level of performance, a finding which has been interpreted as decreased neural efficiency in this group compared to COMT Met carriers (Bertolino et al., 2006; Pomarol-Clotet et al., 2007).
Another potential influence on PFC function is brain-derived neurotrophic factor (BDNF). BNDF is involved in synaptic transmission and plasticity and has been shown to influence hippocampal-dependent learning and memory (Savitz et al, 2006). The BDNF Val66Met polymorphism affects intracellular trafficking of pro-BDNF and secretion of the mature peptide (Egan et al., 2003); carriers of the BDNF Met allele show reduced hippocampal activity during memory encoding and retrieval tasks (Hariri et al., 2003; Hashimoto et al., 2008), and performed worse than BDNF Val homozygotes on a measure of PFC function (Rybakowski et al., 2003). Furthermore, Schofield and colleagues (2009) found that BDNF Met homozygotes showed delays in P300 latency in response to task-relevant target stimuli, which suggests an effect of this polymorphism on selective information processing.
Based on the demonstrated associations of COMT and BDNF with working memory and cognitive processing in the PFC, and the role of cognitive processing and PFC activity in the perceived effectiveness of PSAs, we investigated associations of COMT and BDNF genotype with self-reported message processing, perceived effectiveness, and quitting intentions among smokers after viewing sets of PSAs. We hypothesized that we would see reduced processing and perceived effectiveness of PSAs and lower intentions to quit smoking among individuals carrying the COMT Val or BDNF Met alleles, which have been associated with decreased PFC function. We classified PSAs according to message sensation value (MSV) and argument strength (AS), two factors shown to influence individual responses to PSAs (Strasser et al., 2009), and examined interactions between genotype, MSV, and AS on outcomes.
Methods
Selection of anti-tobacco PSAs
PSA selection and study design were described previously (Strasser et al., 2009). Briefly, 569 cigarette smoking PSAs were acquired from several state and national health authorities. Three trained raters viewed each PSA for content and identified a subset of 99 PSAs that (a) promoted seeking smoking cessation treatment or portrayed the negative consequences of continuing to smoke, (b) targeted adults, and (c) were 30 s in duration. These PSAs were rated for MSV features using a scoring template (visual range = 0 – 10, audio range = 0 – 5, and content range = 0 – 5) based on work by Morgan et al. (2003).
To classify PSAs by argument strength (AS), trained raters viewed the PSAs to generate a single statement reflecting the central argument (or arguments) of each PSA (e.g., “If the health harms of smoking are not enough to get you to quit, consider quitting for your children and those you love,” and “Although you may think smoking helps you cope, if you don't soon quit you will eventually die”). Next, we conducted a shopping mall intercept survey of 300 current smokers to collect ratings of the transcribed central arguments, from which an overall AS score was created for each PSA by taking the mean of the 36-38 individual scores recorded for each PSA (Zhao et al., 2011).
Four groups of PSAs were then created from the existing collection of 99 PSAs: (a) high MSV – high AS, (b) high MSV – low AS, (c) low MSV – high AS, and (d) low MSV – low AS. PSAs exceeding one standard deviation from the mean on each of the two dimensions were selected for use in the present study; 16 PSAs met this criterion (four in each group).
Participant selection
Smokers responding to recruitment flyers and advertisements participated in an initial telephone contact at which eligibility was determined. 199 eligible individuals completed a single 90-minute session. After giving informed consent, participants provided an exhaled breath carbon monoxide sample (Vitalograph; Lenexa, KS) for biochemical verification of smoking status and a saliva sample for genotyping. Of these 199 participants, 122 self-identified as European American (EA, selected to reduce potential bias due to population stratification (Palmatier et al., 1999; Petryshen et al., 2010)). Of these, genotype data (BDNF rs6265 and COMT rs4680) were collected from 120 participants. Genotypes were classified as Val/Val vs. */Met for both BDNF rs6265 and COMT rs4680, based on previous research demonstrating significant cognitive differences between Val homozygotes and Met carriers for both genes (Hariri et al., 2003; Schofield et al., 2009; Loughead et al., 2009; Colzato et al., 2010).
Standard questionnaires (Lerman et al., 1997) were administered at the beginning of the session to assess demographics, smoking history, and nicotine dependence (Fagerström Test for Nicotine Dependence, which includes current smoking rate; Heatherton et al., 1991). Four PSAs were then presented via a 17-inch computer monitor using MediaLab Research Software (Empirisoft, New York, NY) with the participant seated in a comfortable chair approximately one meter away. Each participant viewed one of the four sets of PSAs classified by MSV and AS. After viewing the PSAs, participants completed measures of cognitive processing, narrative processing, and sensory processing of the PSAs (Andrews et al., 1990; Chaudhuri & Buck 1995; Palmgreen et al., 2002); affective response to the PSAs (Batra & Holbrook, 1990; Chaudhuri & Buck 1995); perceived effectiveness of the PSA; recognition of content (Everett & Palmgreen 1995); and intentions to quit smoking (Fishbein et al., 2001; Norman, Conner, & Bell, 1999; Yzer et al., 2003). Items measuring processing were accompanied by response scales ranging from 1 (not at all) to 7 (very much), and assessed thinking about the central message of the PSA (cognitive processing; 5 items, e.g., “Overall, how much did the PSA make you think about arguments for quitting smoking?”), the story told by the PSA (narrative processing; 3 items, e g., “Overall, how much did you pay attention to the characters in the PSA?”), and the sensory qualities of the PSA (sensory processing; 3 items, e g., “Overall, how much did you pay attention to the PSA's sound tracks?”). Perceived effectiveness was measured by four sets of 7 items (one set for each PSA) accompanied by response scales running from 1 (strongly disagree) to 5 (strongly agree). Intentions to quit were measured using two items accompanied by 4-point response scales. Affective response was measured using 12 items assessing emotional response to the PSA (e.g., “Did the PSA make you feel sad?”) accompanied by response scales ranging from -3 (not at all) to 3 (extremely). Participant responses were averaged across all items within each category to generate a summary score for that category, which was used for analysis. Recognition was assessed using five items; for purposes of analysis, a dichotomous recognition variable was used (answered all five items correctly vs. answered at least one item incorrectly).
Descriptive statistics were obtained for all variables. One-way ANOVAs were used to test for differences across genotype groups in demographics, smoking history, quitting intentions, processing, perceived PSA effectiveness, and recognition. Linear regression models of quitting intentions, perceived effectiveness, message processing, and affective response and a logistic regression model of the dichotomized recognition measure were then performed. The predictors were age, nicotine dependence (continuous), education (college graduate = 1, non-college graduate = 0), message sensation value (low = 0, high = 1), argument strength (low = 0, high = 1), and the dichotomous versions of BDNF Val66Met (Val/Val = 0 and */Met = 1) and COMT Val158Met (*/Met = 0 and Val/Val = 1). The two-way interactions of the genotype variables with message sensation value and argument strength were also included. All predictors were entered as a block, after which nonsignificant (p > .05) interaction terms were allowed to drop out.
We investigated the possibility that the association of BDNF genotype with quitting intentions and perceived effectiveness was mediated by central processing using the method originally proposed by Baron & Kenny (1986) and applied to tobacco prevention research by MacKinnon et al. (2002). Using this method, mediation is demonstrated when (a) the predictor is significantly associated with both the outcome and the mediator, and (b) in a regression of outcome on predictor and mediator, the mediator is significantly associated with the outcome (which should reduce, or render nonsignificant, the effect of the predictor.) To accomplish this, regression models of quitting intentions and perceived effectiveness were performed, using BDNF genotype and central processing as predictors.
Results
Demographics
In the final sample of 120 smokers, 69 (57.5%) were male and 40 (33.3%) graduated college. Mean age was 41.52 years (SD = 12.49), mean number of cigarettes smoked each day at baseline was 23.25 (SD = 16.06), and mean score at baseline on nicotine dependence was 5.13 (SD = 2.49).
Genotype Distribution
The genotype distribution for BDNF Val66Met was 75 (62.5%) Val/Val, 40 (33.3%) Val/Met, and 5 (4.2%) Met/Met. For COMT Val158Met the distribution was 29 (24.2%) Met/Met, 72 (60.0%) Val/Met, and 19 (15.8%) Val/Val. Both genotypes were in Hardy-Weinberg equilibrium. For hypothesis testing, both BDNF and COMT genotypes were dichotomized as Val/Val vs. */Met. Characteristics of demographics did not differ by BDNF genotype; however, there were differences in smoking rate and nicotine dependence by COMT genotype (Table 1). No differences were found in the distribution of genotypes across the study conditions.
Table 1. Demographics by Genotype.
| Variable | BDNF Val66Met | COMT Val158Met | ||||
|---|---|---|---|---|---|---|
| Val/Val (n = 75) | Val/Met (n = 40) | Met/Met (n = 5) | Val/Val (n = 19) | Val/Met (n = 72) | Met/Met (n = 29) | |
| Age, M (sd) | 40.96 (12.67) | 42.62 (12.60) | 41.00 (10.20) | 38.16 (12.65) | 43.43 (12.51) | 38.97 (11.82) |
| Female, N (%) | 32 (42.7%) | 15 (37.5%) | 4 (80.0%) | 10 (52.6%) | 26 (36.1%) | 15 (51.7%) |
| College graduate, N (%) | 25 (33.3%) | 15 (37.5%) | 0 (0.0%) | 7 (36.8%) | 23 (31.9%) | 10 (34.5%) |
| Cigarettes per day, M (sd) | 22.09 (14.51) | 25.95 (19.23) | 19.00 (7.42) | 16.05a (7.79) | 25.94a (19.32) | 21.28a (7.30) |
| Nicotine dependence, M (sd) | 5.03 (2.64) | 5.42 (2.23) | 4.40 (2.41) | 3.79b (1.99) | 5.26b (2.63) | 5.69b (2.19) |
F for difference across these three means = 3.26, p = .042.
F for difference across these three means = 3.74, p = .027.
Genetic associations with self-report outcomes
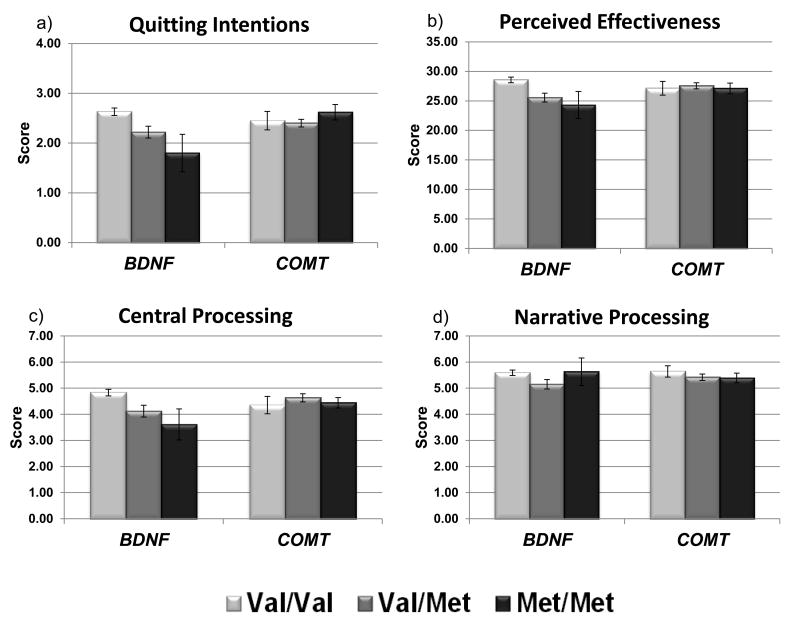
In the multivariate models, BDNF genotype was associated significantly with quitting intentions (β = -0.31, t = -3.38, p = .001), perceived effectiveness (β = -0.32, t = -3.76, p < .001), central processing (β = -0.29, t = -3.31, p = .001), and narrative processing (β = -0.19, t = -2.02, p = .046); as shown in Figure 1, the reduced function BDNF Met allele was associated with lower scores on all of these measures . There were no significant main effects of COMT genotype; however, there was a significant interaction of COMT genotype by argument strength in the model of narrative processing (β = 0.27, t = 2.02, p = .046), indicating that the COMT Val/Val genotype was associated with higher narrative processing scores in the high argument strength condition, but not in the low argument strength condition (where a trivial difference in the opposite direction was observed). Neither BDNF nor COMT genotype contributed to the models of sensory processing, affective response, or recognition.
Figure 1.
Association of outcome measures with genotype. Results are presented as mean scores +/- standard error. In the multivariate models, the dichotomous BDNF genotype (Val/Val vs. */Met) was significantly associated with quitting intentions (β = -0.31, p = .001), perceived effectiveness (β = -0.32, p < .001), central processing (β = -0.29, p = .001), and narrative processing (β = -0.19, p = .046).
As described previously, to test the possibility that central processing might mediate the effect of BDNF genotype on perceived effectiveness and quitting intentions, additional regression models of perceived effectiveness and quitting intentions were performed, using BDNF genotype and central processing as predictors. As shown in Table 2, in both of the models, the effect of central processing was highly significant, and the effect of BDNF genotype was reduced, becoming nonsignificant (p = .076) in the model of perceived effectiveness.
Table 2. Results of Regression Models Testing Mediation of the Effect of BDNF Val66Met Genotype on Quitting Intentions and Perceived Effectiveness by Central Processing.
| Outcome | Effect of: | |||||
|---|---|---|---|---|---|---|
| BDNF Val66Met | Central processing | |||||
| β | t | p | β | t | p | |
| Quitting intentions | -0.18 | -2.12 | .037 | 0.41 | 4.81 | < .001 |
| Perceived effectiveness | -0.10 | -1.79 | .076 | 0.80 | 14.97 | < .001 |
Discussion
We report a significant association of BDNF Val66Met genotype with central processing, narrative processing, and perceived effectiveness of the PSAs used in this study, and with quitting intentions; the reduced function BDNF Met allele was associated with lower scores on all of these measures. We demonstrate that central processing acts as a mediator of the effect of BDNF genotype on quitting intentions and perceived effectiveness; these results are independent upon nicotine dependence.
The BDNF Val66Met polymorphism affects intracellular trafficking and secretion of the mature BDNF peptide; the reduced-function BDNF Met allele impacts measures of memory encoding and retrieval, selective information processing, and general PFC function (Egan et al., 2003; Hariri et al., 2003; Rybakowski et al., 2003; Schofield et al., 2009). Delays in information processing and difficulties in memory encoding may negatively affect an individual's ability to follow and process the central message of a PSA, especially given that the short duration of the PSA requires this information to be processed quickly. It is possible that an individual who experiences more difficulty in processing the central argument may not find the PSA to be effective or persuasive, and thus the PSA would have little effect on quitting intentions.
The BDNF Val66Met polymorphism has also been shown to influence emotional response and emotional decision making (Gasic et al., 2009; Kang et al., 2010), and it is possible that the effects we demonstrate could be influenced by emotional processing of the PSAs used in the study. We did not see an association between BDNF genotype and affective response to the PSAs, and therefore we believe the associations of this polymorphism with quitting intentions and perceived effectiveness in our study are primarily mediated by differences in cognitive processing between BDNF Val/Val homozygotes and Met carriers. However, future studies which more closely examine the influence of BDNF Val66Met on emotional response to PSAs may be of interest.
While we did not see a main effect of COMT Val158Met genotype on the primary outcomes of the study, we did note a significant interaction of COMT genotype by argument strength in the model of narrative processing; individuals homozygous for the COMT Val allele reported higher narrative processing in the high argument strength condition, but not in the low argument strength condition. The COMT Val allele encodes the normally functioning protein; dopamine levels in the PFC of COMT Val homozygotes are lower than in COMT Met allele carriers. Most studies of the COMT Val158Met polymorphism have focused on deficits in working memory in COMT Val homozygotes (Egan et al., 2001; Goldberg et al., 2003). However, a recent study suggests that the rapid regulation of dopamine levels in the COMT Val homozygotes may afford greater cognitive flexibility; COMT Val/Val individuals were faster and more efficient than COMT Met carriers in a task-switching paradigm (Colzato et al., 2010). It is therefore possible that COMT Val homozygotes in our study were able to more rapidly shift cognitive resources toward processing information in a PSA if the argument was strong enough to warrant consideration.
Generally, the effects of argument strength on the outcomes in our models were not as great as we expected. This could be due to the fact that the PSAs selected for this study were actual PSAs designed for, and used in, anti-smoking campaigns. Because no anti-smoking campaign would intentionally use extremely weak arguments as an intervention, the observed variation in argument strength across PSAs (while validated; Zhao et al., 2011) was relatively limited.
A limitation of this study is that we rely on accurate self-assessment of cognitive processing; our measures are not direct or objective measurements of cognitive processing. Despite this, we have no reason to suspect differences in self-report based on genotype, so the likelihood of bias is low, and the measures used here have shown good construct validity in previous research (Andrews et al., 1990; Chaudhuri & Buck 1995; Palmgreen et al., 2002). Another limitation is that time since last cigarette was not controlled. Nicotine administration is known to improve attention (Heishman et al., 2010) and nicotine withdrawal syndrome includes cognitive deficits (Leventhal et al., 2010); inconsistent levels of nicotine withdrawal among our participants may have added to error variance. However, in real-life situations smokers may view PSAs at any time; therefore our findings may more closely reflect a real-world effect. Finally, our sample consisted entirely of non-treatment seeking smokers viewing ads with a smoking cessation theme. Chronic nicotine exposure has been shown to alter levels of BDNF (Czubak et al., 2009; Bhang et al., 2010), which may alter the degree to which BDNF genotype influences cognitive processing. Furthermore, some anti-smoking PSAs employ different themes, such as smoking prevention or emphasizing the dangers of second-hand smoke, and are directed toward other groups (adolescents who have not started smoking or non-smokers). Therefore our results may not apply to all anti-smoking PSAs or target audiences.
In conclusion, we have demonstrated a significant association of BDNF Val66Met genotype with self-report outcomes of cognitive processing independent upon nicotine dependence, and a significant interaction of COMT Val158Met genotype by argument strength on narrative processing. Our findings are particularly interesting in light of recent studies suggesting an association between BDNF and COMT genotypes and smoking. The BDNF Met allele has been associated with increased risk of smoking initiation and nicotine dependence (Beuten et al., 2005; Lang et al., 2007; Novak et al., 2010), although not in all populations (Montag et al., 2008); the COMT Val allele has been associated with smoking status (Nedic et al., 2010) and decreased quit rates in smoking cessation studies (Colilla et al., 2005), possibly mediated by an increased susceptibility to cognitive symptoms following nicotine abstinence (Loughead et al., 2009). Given these associations, further consideration of genetic influences on cognitive processing of anti-smoking PSAs may be useful in order to increase PSA effectiveness for the target population. Moreover, our research may have broader implications concerning health messaging in at-risk populations. Future research into genotype associations with response to other health-behavior messages (for example, healthy eating for obese populations) may provide valuable insights for public health campaigns.
References
- Andrews JC, Durvasula S, Akhter SH. A framework for conceptualizing and measuring the involvement construct in advertising research. J Advertising. 1990;19(4):27–40. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Batra R, Holbrook MB. Developing a typology of affective response to advertising. Psychol Market. 1990;7(1):11–25. [Google Scholar]
- Beuten J, Ma JZ, Payne TJ, Dupont RT, Quezada P, Huang W, et al. Significant association of BDNF haplotypes in European-American male smokers but not in European-American female or African-American smokers. Am J Med Genet B Neuropsychiatr Genet. 2005;139B(1):73–80. doi: 10.1002/ajmg.b.30231. [DOI] [PubMed] [Google Scholar]
- Bhang SY, Choi SW, Ahn JH. Changes in plasma brain-derived neurotrophic factor levels in smokers after smoking cessation. Neurosci Lett. 2010;468(1):7–11. doi: 10.1016/j.neulet.2009.10.046. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26(15):3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Harris JE, Hamilton W. Impact of the Massachusetts tobacco control programme: population based trend analysis. Bmj. 2000;321(7257):351–354. doi: 10.1136/bmj.321.7257.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Buck R. Media differences in rational and emotional responses to advertising. J Broadcast Electron. 1995;39(1):109–125. [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, et al. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nat Neurosci. 2011 doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colilla S, Lerman C, Shields PG, Jepson C, Rukstalis M, Berlin J, et al. Association of catechol-O-methyltransferase with smoking cessation in two independent studies of women. Pharmacogenet Genomics. 2005;15(6):393–398. doi: 10.1097/01213011-200506000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Waszak F, Nieuwenhuis S, Posthuma D, Hommel B. The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val158Met polymorphism: evidence for a role of dopamine in the control of task-switching. Neuropsychologia. 2010;48(9):2764–2768. doi: 10.1016/j.neuropsychologia.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubak A, Nowakowska E, Kus K, Burda K, Metelska J, Baer-Dubowska W, et al. Influences of chronic venlafaxine, olanzapine and nicotine on the hippocampal and cortical concentrations of brain-derived neurotrophic factor (BDNF) Pharmacol Rep. 2009;61(6):1017–1023. doi: 10.1016/s1734-1140(09)70163-x. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Everett M, Palmgreen P. Influences of sensation seeking, message sensation value, and program context on effectiveness of anti-cocaine public service announcements. Health Commun. 1995;7:225–248. [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD. Predicting persuasion-induced behavior change from the brain. J Neurosci. 2010a;30(25):8421–8424. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Rameson L, Berkman ET, Liao B, Kang Y, Inagaki TK, et al. The neural correlates of persuasion: a common network across cultures and media. J Cogn Neurosci. 2010b;22(11):2447–2459. doi: 10.1162/jocn.2009.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein M, Hennessy M, Kamb M, Bolan GA, Hoxworth T, Iatesta M, et al. Using intervention theory to model factors influencing behavior change. Project RESPECT. Eval Health Prof. 2001;24(4):363–384. doi: 10.1177/01632780122034966. [DOI] [PubMed] [Google Scholar]
- Gasic GP, Smoller JW, Perlis RH, Sun M, Lee S, Kim BW, et al. BDNF, relative preference, and reward circuitry responses to emotional communication. Am J Med Genet Part B. 2009;150B:762–781. doi: 10.1002/ajmg.b.30944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psych. 2003;60(9):889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23(17):6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Moriguchi Y, Yamashita F, Mori T, Nemoto K, Okada T, et al. Dose-dependent effect of the Val66Met polymorphism of the brain-derived neurotrophic factor gene on memory-related hippocampal activity. Neurosci Res. 2008;61(4):360–367. doi: 10.1016/j.neures.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JI, Namkoong K, Ha RY, Jhung K, Kim YT, Kim SJ. Influence of BDNF and COMT polymorphisms on emotional decision making. Neuropharmacology. 2010;58:1109–1113. doi: 10.1016/j.neuropharm.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Lang UE, Sander T, Lohoff FW, Hellweg R, Bajbouj M, Winterer G, et al. Association of the met66 allele of brain-derived neurotrophic factor (BDNF) with smoking. Psychopharmacology (Berl) 2007;190(4):433–439. doi: 10.1007/s00213-006-0647-1. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Loughead JW, Ruparel K, Hakun JG, Busch-Winokour S, Holloway MB, et al. Reduced prefrontal and temporal processing and recall of high “sensation value” ads. NeuroImage. 2009;46:219–225. doi: 10.1016/j.neuroimage.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Gold K, Audrain J, Lin TH, Boyd NR, Orleans CT, et al. Incorporating biomarkers of exposure and genetic susceptibility into smoking cessation treatment: effects on smoking-related cognitions, emotions, and behavior change. Health Psychol. 1997;16(1):87–99. doi: 10.1037//0278-6133.16.1.87. [DOI] [PubMed] [Google Scholar]
- Leshner G, Cheng IH. The effects of frame, appeal, and outcome extremity of antismoking messages on cognitive processing. Health Commun. 2009;24(3):219–227. doi: 10.1080/10410230902804117. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addict Behav. 2010;35(12):1120–1130. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, et al. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry. 2009;14(8):820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Taborga MP, Morgan-Lopez AA. Mediation designs for tobacco prevention research. Drug and Alcohol Dependence. 2002;68:S69–S83. doi: 10.1016/s0376-8716(02)00216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer K, Pierce JP, Zhu SH, Hartman AM, Al-Delaimy WK, Trinidad DR, et al. The California Tobacco Control Program's effect on adult smokers: (1) Smoking cessation. Tob Control. 2007;16(2):85–90. doi: 10.1136/tc.2006.016873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Basten U, Stelzel C, Fiebach CJ, Reuter M. The BDNF Val66Met polymorphism and smoking. Neurosci Lett. 2008;442(1):30–33. doi: 10.1016/j.neulet.2008.06.064. [DOI] [PubMed] [Google Scholar]
- Morgan SE, Palmgreen P, Stephenson MT, Lorch EP, Huyle RH. The relationship between message sensation value and perceived message sensation value: The effect of formal message features on subjective evaluations of anti-drug public service announcements. J Commun. 2003;53:512–526. [Google Scholar]
- Nedic G, Nikolac M, Borovecki F, Hajnsek S, Muck-Seler D, Pivac N. Association study of a functional catechol-O-methyltransferase polymorphism and smoking in healthy Caucasian subjects. Neurosci Lett. 2010;473(3):216–219. doi: 10.1016/j.neulet.2010.02.050. [DOI] [PubMed] [Google Scholar]
- Novak G, LeBlanc M, Zai C, Shaikh S, Renou J, DeLuca V, et al. Association of polymorphisms in the BDNF, DRD1 and DRD3 genes with tobacco smoking in schizophrenia. Ann Hum Genet. 2010;74(4):291–298. doi: 10.1111/j.1469-1809.2010.00578.x. [DOI] [PubMed] [Google Scholar]
- Norman P, Conner M, Bell R. The theory of planned behavior and smoking cessation. Health Psychol. 1999;18(1):89–94. doi: 10.1037//0278-6133.18.1.89. [DOI] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Palmgreen P, Stephenson MT, Everett MW, Baseheart JR, Francies R. Perceived message sensation value (PMSV) and the dimensions and validation of a PMSV scale. Health Commun. 2002;14(4):403–428. doi: 10.1207/S15327027HC1404_1. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry. 2010;15:810–815. doi: 10.1038/mp.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol-Klotet E, Fatjό-Vilas M, McKenna PJ, Monté GC, Sarrό S, Ortiz-Gil J, et al. COMT Val158Met polymorphism in relation to activation and de-activation in the prefrontal cortex: a study in patients with schizophrenia and healthy subjects. NeuroImage. 53:899–907. doi: 10.1016/j.neuroimage.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Skibinska M, Hauser J. Polymorphism of the brain-derived neurotrophic factor gene and performance on a cognitive prefrontal test in bipolar patients. Bipolar Disord. 2003;5(6):468–472. doi: 10.1046/j.1399-5618.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5(4):311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Williams LM, Paul RH, Gatt JM, Brown K, Luty A, et al. Disturbances in selective information processing associated with the BDNF Val66Met polymorphism: evidence from cognition, the P300 and fronto-hippocampal systems. Biol Psychol. 2009;80(2):176–188. doi: 10.1016/j.biopsycho.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Siegel M. Mass media antismoking campaigns: a powerful tool for health promotion. Ann Intern Med. 1998;129(2):128–132. doi: 10.7326/0003-4819-129-2-199807150-00013. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Cappella JN, Jepson C, Fishbein M, Tang KZ, Han E, et al. Experimental evaluation of antitobacco PSAs: effects of message content and format on physiological and behavioral outcomes. Nicotine Tob Res. 2009;11(3):293–302. doi: 10.1093/ntr/ntn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Report on the global tobacco epidemic 2009 [Google Scholar]
- Yzer MC, Cappella JN, Fishbein M, Hornik R, Ahern RK. The effectiveness of gateway communications in anti-marijuana campaigns. J Health Commun. 2003;8(2):129–143. doi: 10.1080/10810730305695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Strasser AA, Cappella JN, Lerman C, Fishbein M. A measure of perceived argument strength: reliability and validity in health communication contexts. Communication Methods and Measures. 2011;5(1):48–75. doi: 10.1080/19312458.2010.547822. [DOI] [PMC free article] [PubMed] [Google Scholar]