Figure 3.
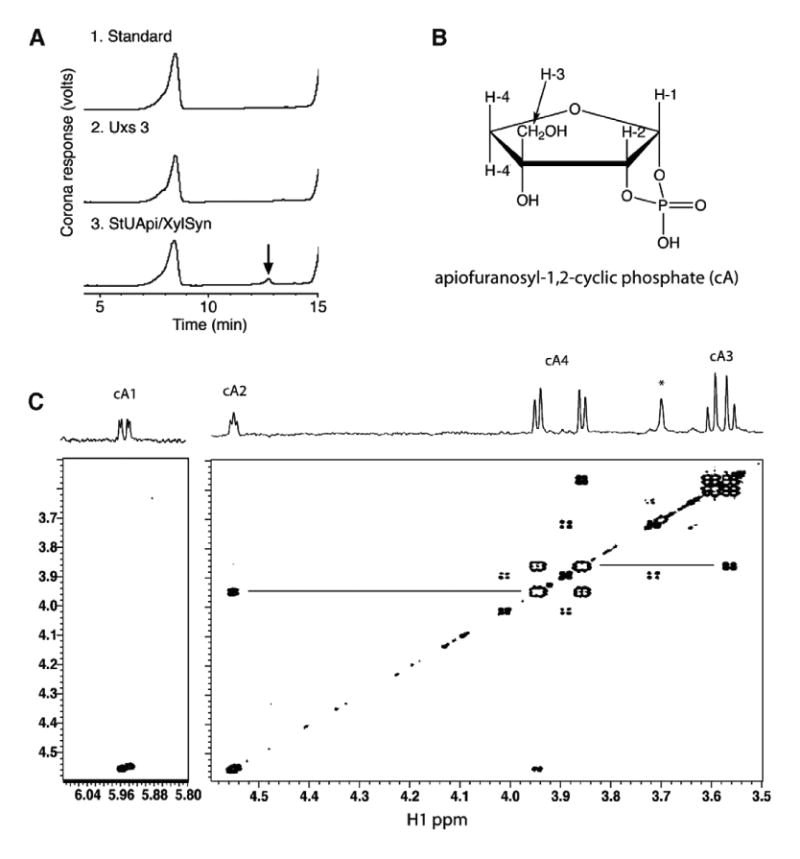
Identification of apiofuranosyl-1,2-cyclic phosphate (cA). Panel A: Ion-exchange HPLC chromatogram of products formed when UDP-GlcA and NAD+ were reacted with the recombinant UAXS. A peak (marked by arrow) was detected when the reaction mixture contained UAXS but was absent in the control reactions. Panel B: Chemical structure: in this numbering scheme the exocyclic methylene protons are labeled as H3, and ring methylene protons are labeled as H4. (C) COSY NMR spectrum: Four-bond couplings between H2 and H4, and H3 and H4, are indicated by horizontal lines. The signals are labeled as cA1, etc. to conform to subsequent figures.
