Figure 2.
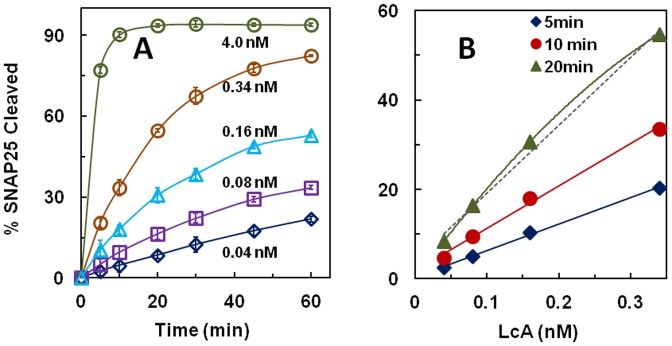
A, Time course of reaction of LcA utilizing the full length SNAP25 (11.3 µM) as a substrate in the presence of 0.2 mg/ml BSA, 0.25 mM ZnCl2 and 5 mM DTT in 50 mM NA-HEPES, pH 7.4 at 37°C. LcA concentrations ranging from 0.04 nM to 4.0 nM were as indicated. Each data point is an average of 5 assays. Bars represent standard deviations. B, 5, 10 and 20-min data for four of the LcA concentrations used in A are plotted as a function of LcA concentration. The dotted line is the best fit (y = mx + c) of the 20-min data points while the curved lines connect the actual data points for each of 5-min, 10-min and 20-min data points. These results show that (a) above 0.04 nM LcA concentration, the time course becomes nonlinear (due to substrate depletion), (b) LcA concentration as low as 0.04 nM remains stable at 37°C for at least 1 hour, and (c) if enough substrate is available, the reaction rate should be linear between 0.04 and 4.0 nM LcA concentration.
