ABSTRACT
BACKGROUND
Home wireless device monitoring could play an important role in improving the health of patients with poorly controlled chronic diseases, but daily engagement rates among these patients may be low.
OBJECTIVE
To test the effectiveness of two different magnitudes of financial incentives for improving adherence to remote-monitoring regimens among patients with poorly controlled diabetes.
DESIGN
Randomized, controlled trial. (Clinicaltrials.gov Identifier: NCT01282957).
PARTICIPANTS
Seventy-five patients with a hemoglobin A1c greater than or equal to 7.5 % recruited from a Primary Care Medical Home practice at the University of Pennsylvania Health System.
INTERVENTIONS
Twelve weeks of daily home-monitoring of blood glucose, blood pressure, and weight (control group; n = 28); a lottery incentive with expected daily value of $2.80 (n = 26) for daily monitoring; and a lottery incentive with expected daily value of $1.40 (n = 21) for daily monitoring.
MAIN MEASURES
Daily use of three home-monitoring devices during the three-month intervention (primary outcome) and during the three-month follow-up period and change in A1c over the intervention period (secondary outcomes).
KEY RESULTS
Incentive arm participants used devices on a higher proportion of days relative to control (81 % low incentive vs. 58 %, P = 0.007; 77 % high incentive vs. 58 %, P = 0.02) during the three-month intervention period. There was no difference in adherence between the two incentive arms (P = 0.58). When incentives were removed, adherence in the high incentive arm declined while remaining relatively high in the low incentive arm. In month 6, the low incentive arm had an adherence rate of 62 % compared to 35 % in the high incentive arm (P = 0.015) and 27 % in the control group (P = 0.002).
CONCLUSIONS
A daily lottery incentive worth $1.40 per day improved monitoring rates relative to control and had significantly better efficacy once incentives were removed than a higher incentive.
KEY WORDS: chronic disease, health information technology, health behavior, disease management, adherence
INTRODUCTION
Health financing in the United States is shifting from a reactive, visit-based system with predominant fee-for-service reimbursement to one that focuses on population health management. For health systems to navigate this transition, cost-effective approaches to managing the health of patients with chronic diseases will be necessary. Ongoing home-based monitoring of health using wireless devices is a promising approach to using technology to augment the capabilities of providers, but its potential as a tool to improve population health is likely to be limited by low patient adherence.1–7 For example, surveys of large employers typically report rates of employee participation in disease management programs of 20 % or lower.8 Given low rates of adherence among many patients with poorly controlled disease, approaches to improve care based on self-monitoring are unlikely to result in substantial improvements in health unless they are accompanied by efforts to enhance patient engagement.
Lottery-based financial incentives that incorporate insights from behavioral economics, such as overweighting of small probabilities, anticipated regret, and loss aversion, have been used successfully to promote healthy behaviors such as weight loss and medication adherence.9–11 In this study, we conduct the first test of the efficacy of lottery-based incentives to promote self-monitoring of blood glucose, blood pressure, and weight among patients with uncontrolled diabetes. Specifically, we test the efficacy of two magnitudes of daily lottery-based financial incentives (expected daily value of $2.80 versus expected daily value of $1.40) on increasing adherence to a wireless monitoring regimen compared to a no-incentive control group.
METHODS
We conducted a 24-week randomized, controlled trial between March 2011 and July 2012, consisting of a 12-week intervention period and a 12-week follow-up period. Seventy-five participants gave their informed consent (Fig. 1) and were randomly assigned to a daily home-monitoring control group or one of two financial incentive groups. All participants were given three biometric devices to use for self-monitoring at home: a OneTouch Ultra glucometer (model Ultra2), an A&D Medical digital blood pressure monitor (model UA-767PBT), and an A&D Medical digital scale (model UC-321PBT). They also received a device that automatically transmitted readings from the biometric devices to the study website (MedApps HealthPAL model MA105). All participants had access to a secure website where they could monitor their measurements. All participants were instructed to use each biometric device once daily (between 12:00 AM and 11:59 PM) and to ensure successful measurement transmission from the HealthPAL to the study website. Participants in the financial incentive arms who used their three devices on a given day were entered into a lottery for a financial incentive on the following day. The protocol was approved by the institutional review board of the University of Pennsylvania.
Figure 1.
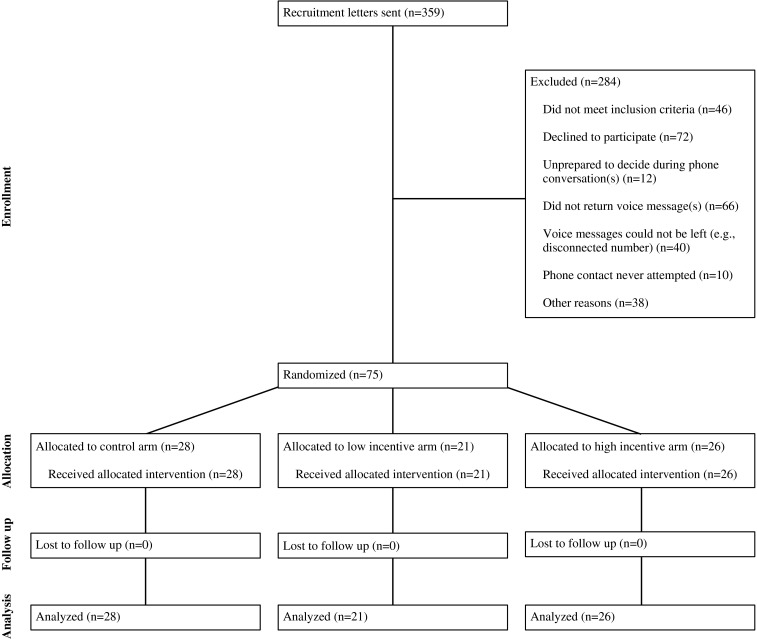
Study flow diagram.
Setting and Participants
Potential participants were identified using a targeted query of electronic medical records of a medical home practice at the University of Pennsylvania Health System. Eligible patients identified by the query were between age 18 and 80 years with a hemoglobin A1c value within six weeks of enrollment that was greater than or equal to 7.5 %, and were able and willing to use either email or a personal cell phone with text messaging capabilities to communicate with the study team (the A1c inclusion criteria was initially set at a value greater than or equal to 8 % within six weeks of enrollment, but was lowered to 7.5 % to accelerate enrollment within the time period required by trial funding). Exclusion criteria included: enrollment in other ongoing clinical trials, diagnosis of an uncontrolled psychiatric disease, and hospitalization in the preceding 12 months with a primary diagnosis of heart failure.
Patients meeting these criteria (n = 359) received a recruitment letter describing the study and inviting them to contact study personnel; study coordinators called patients who did not respond to the letter to inquire about their interest. Of those 359, 84 declined to participate or were undecided, 46 did not meet inclusion criteria upon further questioning, and 106 could not be reached by telephone. Of the remaining 123 patients, 48 were excluded for other reasons and 75 were enrolled in-person by the study coordinator between March 2011 and January 2012. At the enrollment visit, participants provided informed consent, blood pressure, blood glucose, and weight measurements were taken, and participants completed a survey of their demographic information and health behaviors.
Randomization and Interventions
The study was conducted using “Way to Health,” an automated information technology platform based at the University of Pennsylvania that integrates biometric devices, clinical trial randomization and enrollment processes, financial system fulfillment, and secure data capture for research purposes.12 At enrollment, participants were electronically randomized in equal proportions using a random number sequence to one of three study arms: two that offered lottery-based financial incentives of different magnitudes based on daily device use during the intervention period (n = 21 in the low incentive group, n = 26 in the high incentive group), and a no-incentives control arm (n = 28). Participants randomized to either incentive arm were asked to pick a number between 00 and 99, and were informed of the study’s daily lottery process in which the study website randomly generated a winning two-digit number each morning. In the high incentive arm, a two-digit match (1 in 100 chance) yielded a $100 reward and a single-digit match in either digit position (1 in 5 chance) yielded a $10 reward. The expected average daily reward was therefore $2.80, since participants could not receive both rewards on the same day. In the low incentive arm, rewards were $50 and $5, respectively, for an expected average daily reward of $1.40. Incentive arm participants were informed that they would be eligible for these winnings only if they had used all three biometric devices and successfully transmitted their results to the study website the day before.
Each day, all incentive arm participants were sent a text message, an email, or both (according to individual preferences set at enrollment) informing them of that morning’s winning lottery number. Eligible participants with either a one-digit or two-digit match were also specifically notified of their reward. Ineligible participants with a match were specifically notified that they would have won had they used their devices the previous day, drawing on research showing that the desire to avoid regret can be motivating.13–16 Participants in both incentive arms were unaware that there was another incentive arm with different lottery amounts, and participants in the control arm were unaware that some participants were eligible for incentives.
In addition to the enrollment visit, all participants attended visits with the study coordinator at month 3 (end of intervention) and month 6 (end of follow-up period). Both visits included repeat blood pressure and weight measurements and surveys; at the month 6 visit, devices were returned. Participants were paid $25 for each visit, as well as $25 for returning the devices. A lab order was provided for a repeat hemoglobin A1c measurement at the month 3 visit only. Throughout the study, all personnel were blinded to participant arm assignments, with the exception of the study coordinator, who administered the distribution of rewards. No participants were lost to follow-up. In addition to participants having access to their measurements online, device readings were shared with a clinician at the practice at which the study was based. If participant measurements were deemed medically dangerous according to pre-established criteria, an alert was sent automatically to the clinician via email for follow-up.
Outcomes and Statistical Analysis
Our primary outcome was adherence (daily use of all biometric devices) during the intervention period (months 1–3). Our secondary outcomes were adherence during the three-month post-intervention period (months 4–6), hemoglobin A1c change from baseline to month 3, and daily use of any biometrics devices (one or more) during both the intervention and follow-up periods. We hypothesized that participants in both incentive arms would have greater adherence rates than participants in the control arm, and that participants in the high incentive arm would have higher rates than those in the low incentive arm.
A priori, we estimated that a sample size of 75 participants (25 per arm) would ensure 80 % power to detect differences between each of the incentive arms and the control arm, using a conservative bonferroni adjustment for multiple comparisons to conserve the overall Type I error rate. This calculation assumed use of all devices on 40 % of days in the control arm compared to 85 % in both the high and low incentive arms using a two-sample comparison of proportions (high incentive versus control and low incentive versus control). We used generalized estimating equations (GEE) to adjust for the repeated-measures nature of the study design.17,18 Models included main effects for arm and month, and arm × month interaction terms to allow for different intervention effects in each month of the study. The dependent variable was the monthly adherence rate, constructed by summing the number of days in each month that all three devices were used and dividing by the number of days in the month. All analyses were performed using SAS (r) Proprietary Software 9.3.
RESULTS
The mean age of participants was 54 years, and 64 % were women. At baseline, about 41 % of participants self-reported low rates of adherence to diabetes medication, 32 % reported medium adherence, and 27 % reported high adherence.19 Average baseline body mass index (BMI), systolic blood pressure, diastolic blood pressure, and hemoglobin A1c values were 34 kg/m2, 133 mmHg, 86 mmHg, and 9.5 %, respectively. There were no significant differences in participants’ baseline characteristics across the three arms (Table 1).
Table 1.
Characteristics of the Study Sample
| Participant Characteristics | Entire Sample (n = 75) | Control (n = 28) | Low-incentive (n = 21) | High-incentive (n = 26) |
|---|---|---|---|---|
| Mean age (SE), y | 54.3 (1.1) | 54.1 (2.0) | 54.7 (2.1) | 54.3 (1.9) |
| Female, n (%) | 48 (64) | 17 (61) | 14 (67) | 17 (65) |
| Race/ethnicity, n (%) | ||||
| White, non-Hispanic | 20 (27) | 7 (25) | 6 (29) | 7 (27) |
| African American, non-Hispanic | 50 (67) | 21 (75) | 14 (67) | 15 (58) |
| Other | 5 (7) | 0 (0) | 1 (5) | 4 (15) |
| Education, n (%) | ||||
| Less than college | 24 (32) | 6 (21) | 6 (29) | 12 (46) |
| Some college | 25 (33) | 11 (39) | 9 (43) | 5 (19) |
| College graduate | 16 (21) | 6 (21) | 5 (24) | 5 (19) |
| Post-college degree | 10 (13) | 5 (18) | 1 (5) | 4 (15) |
| Federal poverty level (FPL), n (%)* | ||||
| < 100 % FPL | 11 (16) | 0 (0) | 4 (20) | 7 (28) |
| 100 % to 199 % FPL | 17 (24) | 11 (42) | 2 (10) | 4 (16) |
| 200 % to 299 % FPL | 13 (18) | 4 (15) | 4 (20) | 5 (20) |
| 300 % to 399 % FPL | 6 (9) | 2 (8) | 2 (10) | 2 (8) |
| ≥ 400 % FPL | 24 (34) | 9 (35) | 8 (40) | 7 (28) |
| Employment, n (%) | ||||
| Employed | 50 (67) | 20 (71) | 14 (67) | 16 (62) |
| Retired | 14 (19) | 6 (21) | 6 (29) | 2 (8) |
| Other | 11 (15) | 2 (7) | 1 (5) | 8 (31) |
| Baseline self-reported medication adherence, n (%) | ||||
| High | 20 (27) | 7 (25) | 6 (29) | 7 (27) |
| Medium | 24 (32) | 9 (32) | 7 (33) | 8 (31) |
| Low | 31 (41) | 12 (43) | 8 (38) | 11 (42) |
| Baseline health characteristics | ||||
| Mean BMI (SE), kg/m2 | 34 (0.8) | 33.6 (1.4) | 34.1 (1.7) | 34.5 (1.1) |
| Mean A1c (SE), % | 9.5 (0.2) | 9.3 (0.3) | 9.3 (0.4) | 9.8 (0.3) |
| Mean SBP (SE), mmHg | 132.9 (2.3) | 128.4 (3) | 136.1 (5.2) | 135.1 (3.8) |
| Mean DBP (SE), mmHg | 86.1 (1.3) | 83.3 (1.9) | 86.3 (2.1) | 88.8 (2.3) |
Frequencies/percentages (categorical variables) compared using χ2-Tests or Fisher’s Exact Test and means (continuous variables) compared using ANOVA or non-parametric Kruskal-Wallis Tests as appropriate. Percentages may not add up to 100 % due to rounding.
BMI body mass index; A1c hemoglobin A1c; SBP systolic blood pressure; DBP diastolic blood pressure.
*FPL data have the following total sample sizes due to missing data: entire sample (n = 71), control (n = 26), low incentive (n = 20), high incentive (n = 25).
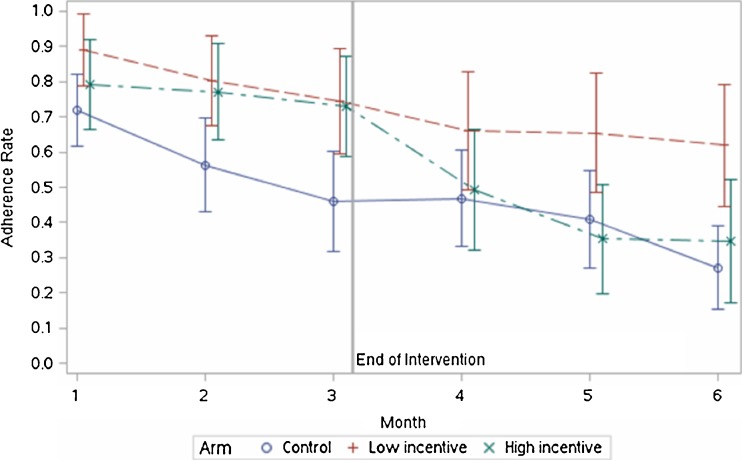
During the three-month intervention period, total adherence rates in both incentive arms were significantly higher than in the control arm (81 % in the low incentive arm versus 58 %, P = 0.007; 77 % in the high incentive arm versus 58 %, P = 0.02). There was no difference in adherence between the two incentive arms during the intervention period (P = 0.58) (Fig. 2). While both incentive arms maintained adherence rates around 80 % throughout the intervention period, rates in the control arm started relatively high at the beginning of the study (perhaps due to the novelty of the devices or a Hawthorne effect) but declined steadily from greater than 70 % in month 1 to less than 50 % in month 3.
Figure 2.
Monthly adherence rate (all device use) by arm. Error bars indicate 95 % CIs.
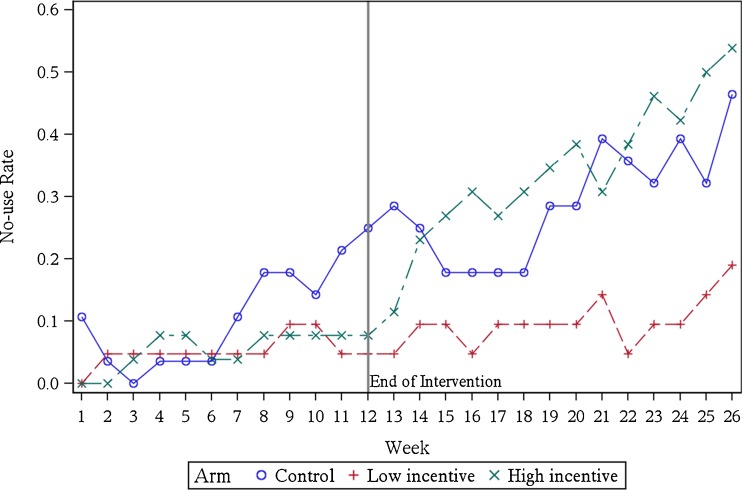
In the subsequent three-month period following the withdrawal of incentives in the intervention arms, adherence in the control arm continued to decline, falling to 27 % by month 6. Adherence in the high incentive arm fell dramatically in month 4 (from 73 % in month 3 to 49 % in month 4), and was no different than the control arm in months 4 (49 % versus 47 %, P = 0.81), 5 (35 % versus 41 %, P = 0.57), and 6 (35 % versus 27 %, P = 0.46). Monthly adherence in the low incentive arm, however, remained significantly higher than the control arm in months 5 (65 % versus 41 %, P = 0.03) and 6 (62 % versus 27 %, P = 0.002). Many participants in the high incentive arm stopped using any of the three devices after incentives were withdrawn at the end of month 3 (Fig. 3). The rate of no device use jumped from 11.5 % in the high incentive arm in the first week following incentive withdrawal to around 50 % by months 5 and 6, while remaining steady around 5–10 % for the majority of the period in the low incentive arm. “No use” rates also rose over time in the control arm.
Figure 3.
Weekly “no device use” rate by arm. “No-use rate” shows the percentage of participants not using any devices in a given week.
Results were qualitatively similar when we used adjusted regression analysis (GEE) to estimate differences between arms in each month, controlling for direct and interaction effects of arm and month (Table 2). There were no significant differences in adherence rates across demographic sub-groups. The majority of incentive arm participants (24 of 26 in the high incentive arm and 20 of 21 in the low incentive arm) won at least one lottery during the intervention period. In the high incentive arm, 92.3 % of participants won the one-digit-match lottery at least once and 61.5 % won the two-digit-match lottery at least once; in the low incentive arm, the percentages were 95.2 % and 52.4 %, respectively. Overall, participants in the high incentive arm earned an average of $188.5 (SD $110.2) in lottery winnings, compared to $93.1 (SD $45.1) in the low incentive arm.
Table 2.
Model-Based Estimates of Differences in Adherence Rates Across Arms and Time
| Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | |
|---|---|---|---|---|---|---|
| A. Adherence (all device use each day) | ||||||
| Monthly Difference Estimate | ||||||
| (95 % CI) | ||||||
| Low incentive vs. control | 0.17* | 0.24** | 0.29** | 0.19 | 0.24* | 0.35*** |
| (0.04, 0.31) | (0.07, 0.41) | (0.09, 0.48) | (−0.01, 0.39) | (0.04, 0.45) | (0.15, 0.54) | |
| High incentive vs. control | 0.07 | 0.21* | 0.27** | 0.03 | −0.06 | 0.08 |
| (−0.08, 0.23) | (0.03, 0.39) | (0.08, 0.46) | (−0.18, 0.23) | (−0.25, 0.14) | (−0.12, 0.27) | |
| Low incentive vs. high incentive | 0.10 | 0.03 | 0.01 | 0.17 | 0.30** | 0.27* |
| (−0.06, 0.25) | (−0.14, 0.21) | (−0.18, 0.21) | (−0.06, 0.39) | (0.09, 0.51) | (0.04, 0.50) | |
| Intervention and Follow-up Period Average Difference Estimates | ||||||
| (95 % CI) | ||||||
| Intervention Period (months 1–3) | Follow-up Period (months 4–6) | |||||
| Low incentive vs. control | 0.23** | 0.26* | ||||
| (0.07, 0.40) | (0.05, 0.47) | |||||
| High incentive vs. control | 0.19* | 0.01 | ||||
| (0.03, 0.34) | (−0.18, 0.21) | |||||
| Low incentive vs. high incentive | 0.05 | 0.25* | ||||
| (−0.12, 0.22) | (0.04, 0.46) | |||||
| B. Adherence (any device use each day) | ||||||
| Monthly Difference Estimate | ||||||
| (95 % CI) | ||||||
| Low incentive vs. control | 0.09 | 0.18* | 0.26** | 0.16 | 0.25* | 0.30** |
| (−0.02, 0.21) | (0.03, 0.33) | (0.09, 0.44) | (−0.02, 0.35) | (0.06, 0.45) | (0.10, 0.49) | |
| High incentive vs. control | 0.07 | 0.16* | 0.21* | −0.02 | −0.05 | −0.01 |
| (−0.04, 0.18) | (0.01, 0.31) | (0.03, 0.39) | (−0.22, 0.18) | (−0.27, 0.16) | (−0.22, 0.21) | |
| Low incentive vs. high incentive | 0.02 | 0.02 | 0.06 | 0.18 | 0.30** | 0.30** |
| (−0.10, 0.14) | (−0.12, 0.16) | (−0.10, 0.21) | (−0.02, 0.39) | (0.10, 0.51) | (0.09, 0.52) | |
| Intervention and Follow-up Period Average Difference Estimates | ||||||
| (95 % CI) | ||||||
| Intervention Period (months 1–3) | Follow-up Period (months 4–6) | |||||
| Low incentive vs. control | 0.18* | 0.24* | ||||
| (0.03, 0.32) | (0.03, 0.44) | |||||
| High incentive vs. control | 0.15* | −0.03 | ||||
| (0.01, 0.28) | (−0.22, 0.17) | |||||
| Low incentive vs. high incentive | 0.03 | 0.26* | ||||
| (−0.11, 0.18) | (0.05, 0.47) | |||||
Difference estimates from GEE regression models, including month, arm, and month*arm interactions
Low incentive arm n = 21, high incentive arm n = 26, control arm n = 28
*p value < 0.05, **p < 0.01, ***p < 0.001
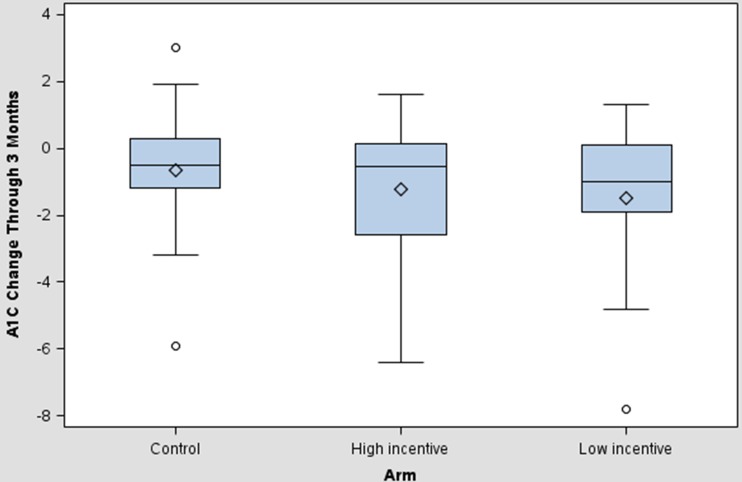
We observed decreases in average A1c levels between enrollment and the end of the intervention period among participants in all groups, with decreases in the low [−1.5 %; 95 % CI (−2.4, -0.5)] and high [−1.2 % (−2.1, -0.3)] incentive arms that were no larger than the control arm [−0.7 % (−1.4, 0.1), P = 0.17 and 0.33, respectively] (Fig. 4). The change in systolic blood pressure was −8.52 mmHg (−18.50, 1.45) in the low incentive arm and 4.17 mmHg (−1.78, 10.12) in the high incentive arm versus 5.19 mmHg (−3.60, 13.98) in the control arm; none of these differences was statistically significant, but greater device usage during the intervention period was significantly correlated with lower blood pressure at month 3 (systolic pressure correlation = −0.31; P = 0.009; diastolic pressure correlation = −0.30; P = 0.01). Point estimates of BMI changes were not statistically significant and close to zero in magnitude. All tests of differences in changes in biometric outcomes between arms were based on one-way Analysis of Variance (implemented in PROC GLM in SAS).
Figure 4.
Box Plot of A1c changes from baseline to end of intervention period. Plots show mean change (and associated 95 % CIs) in A1c level between enrollment and the end of the intervention period by arm.
DISCUSSION
This was the first study to test the impact of lottery-based incentives on adherence to home-based wireless device monitoring for chronic disease management. The study has two main findings. First, we found that both smaller and larger lottery-based incentives were significantly more effective than control at increasing adherence during the intervention period. These results build on and reinforce findings from earlier studies that have demonstrated the success of daily lotteries with expected average daily rewards of $2.80 in motivating weight loss and medication adherence over three to six months of intervention.9–11 Given the steady decline in monthly adherence among control arm participants to usage on 27 % of days by month 6, some sort of incentive intervention could be necessary for such remote monitoring technologies to be used successfully among patients with poor chronic disease management. Because we found similar efficacy in the incentive arm with a daily expected value of $2.80 and the arm with an expected value of $1.40 during the intervention period, our results suggest that within this range, the size of the incentive is less important than the structure of the incentive while incentives are in place.
Second, we found that although adherence waned after the intervention period in both incentive arms, the smaller expected value lottery was considerably more effective in the post-incentives period than the larger expected value lottery. Our finding that performance diminished following the removal of incentives is consistent with other work on the use of incentives for health behavior change.20,21 The divergence of the adherence rates between the two incentive arms post-intervention is of particular interest and has important implications for maintaining behavior change once incentives are removed. One possible explanation for the dramatic drop in adherence among those in the high incentive arm may be a “negative contrast effect” that made the loss of incentive especially salient to those previously receiving the relatively higher incentive, leading to an adverse emotional response and decreased adherence.22 Another possibility is that the larger incentive “crowded out” participants’ intrinsic motivation to a greater degree than the smaller incentive, leading these participants to feel that they were adhering not for the sake of their health, but rather for the payment. In turn, once the payment was removed, they may not have felt motivated to adhere.23–26 It is possible that crowding out contributed to the differential behaviors between the two incentive arms. We are, however, unable to quantify this contribution since we did not measure daily adherence prior to introduction of incentives and for full crowding out to be confirmed, adherence would need to have fallen below pre-incentive levels. If this effect is confirmed in future research, it points to a potential pitfall of an incentive program with rewards that create too great a contrast with the post-reward state.
This study is subject to limitations. First, participants came from a single primary care practice, and although the sample was diverse along multiple dimensions, results might not be generalizable to other populations. Second, we deployed incentives for only three months and therefore cannot know how adherence may have changed if incentives had been in place for a longer period. This limitation is important since management of these chronic conditions is generally life-long. Another potential concern is that the population likely to benefit from remote monitoring may lack access to or be reluctant to use technologies such as the internet, thereby limiting the potential applicability of our findings. While it is hard to determine what proportion of adults may face this type of barrier, it is likely that the proportion will decrease with the proliferation of remote monitoring devices with their own communications capabilities. Finally, this small study was designed to measure the effects of incentives on daily use of remote monitoring devices, with changes in health a secondary outcome. Thus, we were not powered to detect any changes in health outcomes across arms, leaving us unable to provide any conclusive evidence of the effect of the intervention on health outcomes.
Increasing patient engagement among populations with high rates of non-adherence is a fundamental challenge of population health management. Wireless technologies that can provide a kind of “automated hovering” offer considerable promise in this area, in part because they may be less expensive and allow for easier daily monitoring and feedback than approaches involving clinical personnel.1 For these technologies to be effective, however, they must be paired with approaches that create and maintain engagement. Our study demonstrates that a daily lottery incentive worth an average of $1.40 per day was associated with significantly greater device usage than in a control group receiving no financial incentives, and was largely free of the substantial drop in post-incentive adherence seen with an incentive double that size. Future work should explore additional ways in which data collected through remote monitoring can be used to improve patient engagement (e.g., using remotely-collected data to set health goals and track performance). In addition, it will be useful to study the cost effectiveness of lotteries of different magnitudes to determine whether still smaller incentives can be used to increase adherence to self-monitoring regimens, and to gain insight into the issue of behavior maintenance upon withdrawal of incentives.
Acknowledgements
Contributors
We thank Paula Gray, MSN, CRNP and Amanda Parent, MSN, CRNP for their participation in and support of this study.
Funders
This work was supported by grants RC2AG036592 and P30AG034546 from the National Institute on Aging.
Prior presentations
We presented an earlier version of this paper on a research panel at AcademyHealth’s 2013 Annual Research Meeting in Baltimore, MD in June, 2013.
Conflict of Interest
The authors declare that they do not have a conflict of interest. Drs. Asch, Loewenstein, and Volpp have served as consultants for VAL Health, and Drs. Loewenstein and Volpp have served as consultants for CVS Caremark. Dr. Volpp has also received research funding from Weight Watchers, CVS Caremark, Humana, Horizon BCBS, and McKinsey, none of which is directly related to the subject of this study. Dr. Loewenstein has also received research funding from CVS Caremark and Humana. The other authors (Sen, Sewell, Riley, Stearman, Bellamy, Hu, Park, Tao, and Zhu) have no financial disclosures or other conflicts of interest to report.
Footnotes
Clinicaltrials.gov Identifier: NCT01282957.
REFERENCES
- 1.Asch DA, Muller RW, Volpp KG. Automated hovering in health care: watching over the 5,000 hours. N Engl J Med. 2012;367:1–3. doi: 10.1056/NEJMp1203869. [DOI] [PubMed] [Google Scholar]
- 2.Miliard M. Automated at-home monitoring lowers high blood pressure, study finds. Available at: http://www.healthcareitnews.com/news/automated-home-monitoring-lowers-high-blood-pressure-study-finds. Accessed January 13, 2014.
- 3.Russell-Minda E, Jutai J, Speechley M, Bradley K, Chudyk A, Petrella R. Health technologies for monitoring and managing diabetes: a systematic review. J Diabetes Sci Technol. 2009;3(6):1460–71. doi: 10.1177/193229680900300628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg LR, Piette JD, Walsh MN, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J. 2003;146(4):705–12. doi: 10.1016/S0002-8703(03)00393-4. [DOI] [PubMed] [Google Scholar]
- 5.Schwedes U, Siebolds M, Mertes G, SMBG Study Group Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care. 2002;25(11):1928–32. doi: 10.2337/diacare.25.11.1928. [DOI] [PubMed] [Google Scholar]
- 6.de Lusignan S, Wells S, Johnson P, Meredith K, Leatham E. Compliance and effectiveness of 1 year’s home telemonitoring. The report of a pilot study of patients with chronic heart failure. Eur J Heart Fail. 2001;3(6):723–30. doi: 10.1016/S1388-9842(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 7.Davidson MB, Castellanos M, Kain D, Duran P. The effect of self-monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: a blinded, randomized trial. Am J Med. 2005;118(4):422–5. doi: 10.1016/j.amjmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Nyce S. Boosting wellness participation without breaking the bank. Towers Watson Insider. Available at http://www.towerswatson.com/en/Insights/Newsletters/Americas/Insider/2010/boosting-wellness-participation-without-breaking-the-bank. Accessed January 13, 2014.
- 9.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631–7. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpp KG, Loewenstein G, Troxel AB, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8:272. doi: 10.1186/1472-6963-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmel SE, Troxel AB, Loewenstein G, et al. Randomized trial of lottery-based incentives to improve warfarin adherence. Am Heart J. 2012;164(2):268–74. doi: 10.1016/j.ahj.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asch DA, Volpp KG. On the Way to Health. LDI Issue Brief. July/August 2012. Volume 17, Issue 9. [PubMed]
- 13.Chapman GB, Coups EJ. Emotions and preventive health behavior: worry, regret, and influenza vaccination. Health Psychol. 2006;25(1):82–90. doi: 10.1037/0278-6133.25.1.82. [DOI] [PubMed] [Google Scholar]
- 14.Zeelenberg M, Pieters R. Consequences of regret aversion in real life: the case of the Dutch postcode lottery. Organ Behav Hum Decis Process. 2004;93:155–68. doi: 10.1016/j.obhdp.2003.10.001. [DOI] [Google Scholar]
- 15.Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–91. doi: 10.2307/1914185. [DOI] [Google Scholar]
- 16.Connolly T, Butler DU. Regret in economic and psychological theories of choice. J Behav Decis Mak. 2006;19(2):148–58. doi: 10.1002/bdm.510. [DOI] [Google Scholar]
- 17.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 18.Zeger SL, Liang KY. The analysis of discrete and continuous longitudinal data. Biometrics. 1986;42:121–30. doi: 10.2307/2531248. [DOI] [PubMed] [Google Scholar]
- 19.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10(5):348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.John LK, Loewenstein G, Troxel AB, Norton L, Fassbender JE, Volpp KG. Financial incentives for extended weight loss: a randomized, controlled trial. J Gen Intern Med. 2011;26(6):621–6. doi: 10.1007/s11606-010-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kullgren JT, Troxel AB, Loewenstein G, et al. Individual- versus group-based financial incentives for weight loss: a randomized, controlled trial. Ann Intern Med. 2013;158(7):505–14. doi: 10.7326/0003-4819-158-7-201304020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberger R, Cameron J. Detrimental effects of reward: reality or myth? Am Psychol. 1996;51(11):1153–66. doi: 10.1037/0003-066X.51.11.1153. [DOI] [PubMed] [Google Scholar]
- 23.Deci EL, Ryan RM. Intrinsic Motivation and Self-Determination in Human Behavior. New York, NY: Plenum Press; 1985. [Google Scholar]
- 24.Lepper MR, Greene D, Nisbett RE. Undermining children’s intrinsic interest with extrinsic reward. J Pers Soc Psychol. 1973;28(1):129–37. doi: 10.1037/h0035519. [DOI] [Google Scholar]
- 25.Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627–68. doi: 10.1037/0033-2909.125.6.627. [DOI] [PubMed] [Google Scholar]
- 26.Gneezy U, Meier S, Rey-Biel P. When and why incentives (don’t) work to modify behavior. J Econ Perspect. 2011;25(4):1–21. doi: 10.1257/jep.25.4.191. [DOI] [Google Scholar]