Figure 1.
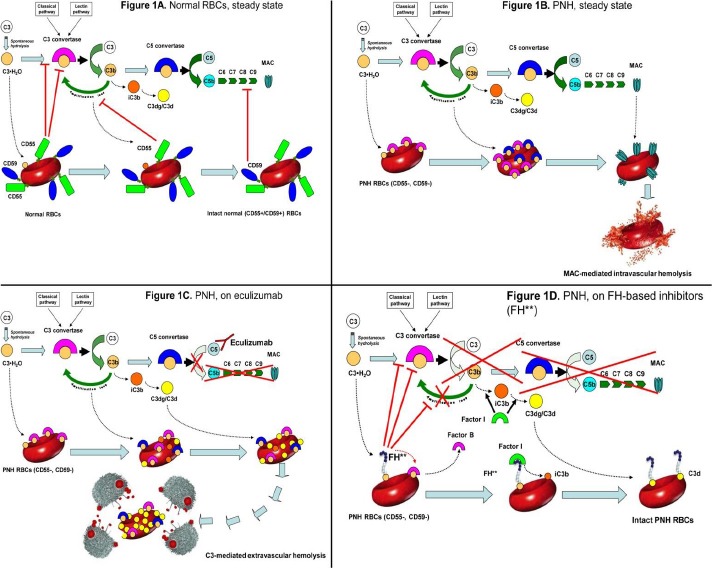
Complement cascade modulation on normal and PNH red blood cells (RBCs), with or without complement inhibitors.
A. Normal RBCs: steady state.
Normal RBCs (CD55+, CD59+) are protected from complement activation by CD55, which down-regulate the C3-convertase, and by CD59, which inhibits the MAC assembly. Thus, in steady state normal RBCs can withstand the challenge of complement activation.
B. PNH RBCs: steady state.
Given their deficiency in surface CD55, PNH RBCs are susceptible to complement activation through C3 tick-over and the alternative pathway, as well as through any other complement pathway. After initial C3 activation and subsequent C3b-binding to erythrocyte surface, the complement cascade may proceed toward the MAC formation, which is not inhibited due to the lack of CD59. As a result, PNH RBCs succumb because of MAC-mediated intravascular hemolysis.
C. PNH RBCs: effect of eculizumab.
Eculizumab binds to C5 in the fluid phase and prevents its cleavage to C5a and C5b. As a result, the progression of the complement cascade toward the MAC assembly is completely inhibited, and PNH RBCs result protected from MAC-mediated intravascular hemolysis. However, given the lack of CD55, initial CAP activation through the C3 tick-over and the CAP-mediated amplification loop remain uncontrolled; thus, early C3 activation continues, and because of their increased survival PNH RBCs progressively bind substantial amount of C3 fragments on their surface. Subsequently, C3-opsonized PNH RBCs become susceptible to C3-receptor mediated clearance by the hepato-splenic reticulo-endothelial system, eventually resulting in C3-mediated extravascular hemolysis.
D. PNH RBCs: effect of C3 inhibitors.
FH-based proteins (FH**), either TT30 or mini-FH, bind to C3b initially activated on PNH RBCs, and deliver their complement-modulatory effect via their FH domain. Thus, FH-based proteins disable the C3-convertase, and, as co-factor of factor I, they also promote the conversion of active C3b into iC3b and then C3dg/C3d, preventing all downstream events due to the complement cascade (as well as additional complement activation on the red cell membrane). As a result, irrespective of the blockade of C5, C3 inhibitors may confer to PNH RBCs a normal survival even in presence of complement activation. All other agents acting at the level of C3 convertase (e.g., compstatin analogs) may work in a similar way, inhibiting initial complement activation on PNH erythrocyte surface and preventing all the downstream events leading to either intravascular or extravascular hemolysis.
“This figure is modified from the research originally published in Blood. Risitano AM, Notaro R, Pascariello C, Sica M, Del Vecchio L, Horvath CJ, Fridkis-Hareli M, Selleri C, Lindorfer MA, Taylor RP, Luzzatto L, Holers VM. The complement receptor 2/factor H fusion protein TT30 protects paroxysmal nocturnalhemoglobinuria erythrocytes from complement-mediated hemolysis and C3 fragment opsonization. Blood. 2012;119(26):6307-16. © the American Society of Hematology.”
