Figure 4. brlR expression and BrlR-DNA binding are enhanced by the secondary messenger c-di-GMP.
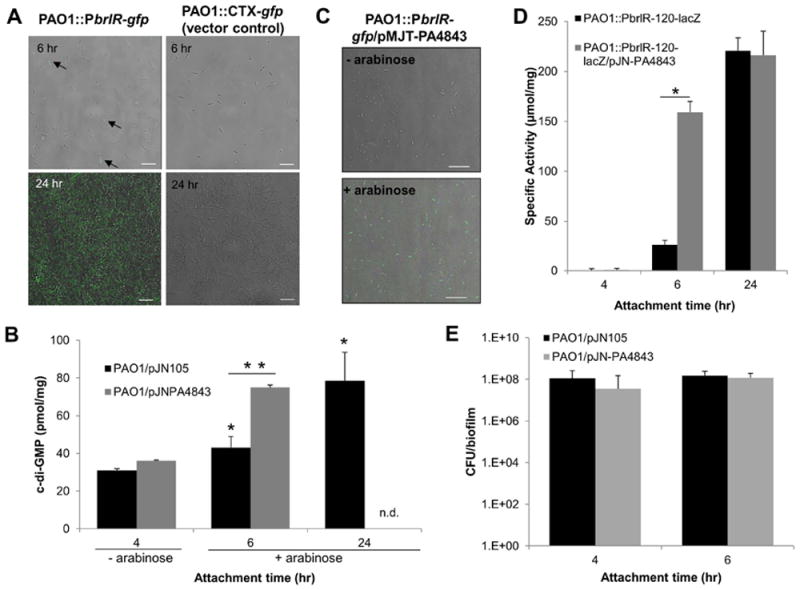
(A) Induction of brlR expression over the course of biofilm formation was monitored by confocal microscopy using a P. aeruginosa PAO1 carrying chromosomal gfp fusions to the upstream region of brlR. Total cells were visualized by brightfield microscopy. Overlays acquired 6 hr and 24 hr post initial attachment are shown. brlR expression was detected as early as 6 hr following initial attachment. Arrows indicate gfp-expressing cells. P. aeruginosa harboring an empty pMini-CTX-gfp vector was used as control. White bar = 10 μm. (B) c-di-GMP levels present in P. aeruginosa harboring an empty pJN105 vector or overexpressing PA4843 (PAO1/pJN-PA4843). The strains were grown attached in tube reactors under flowing conditions for 4, 6, and 24 hr. – arabinose, no arabinose was added to the growth medium. + arabinose, arabinose was added to the growth medium 4 hr post initial attachment to induce PA4843 expression. n.d., not determined. *, significantly different from PAO1/pJN105 at the 4-hr time point; p < 0.05. **, significantly different from PAO1/pJN105 at the 6-hr time point; p < 0.01. (C) Expression of the diguanylate cyclase PA4843 results in enhanced gfp expression indicative of brlR expression, compared to untreated controls. – arabinose, no arabinose was added to the growth medium. + arabinose, arabinose was added to the growth medium 4 hr post initial attachment to induce PA4843 expression. (D) brlR transcriptional reporter activity during surface-attached growth is enhanced upon overexpression of the cyclase PA4843. Strains harboring the brlR-lacZ reporter construct in the absence or presence of the plasmid pJN-PA4843 were grown under continuous flowing conditions in flow cells for 4, 6 hr, and 24 hr. Expression of PA4843 was induced by the addition of arabinose to the growth medium 4 hr post initial attachment. β-galactosidase activity has been corrected by the activity observed for the vector control strain. *, significantly different from PAO1/pJN105 at the 6-hr time point; p < 0.05. (E) Number of viable cells of PAO1/pJN105 and PAO1/pJN-PA4843 attached to glass following 4 and 6 hr post initial attachment under flowing conditions, expressed as CFU/biofilm. All experiments were carried out in triplicate. Error bars indicate standard deviations.
