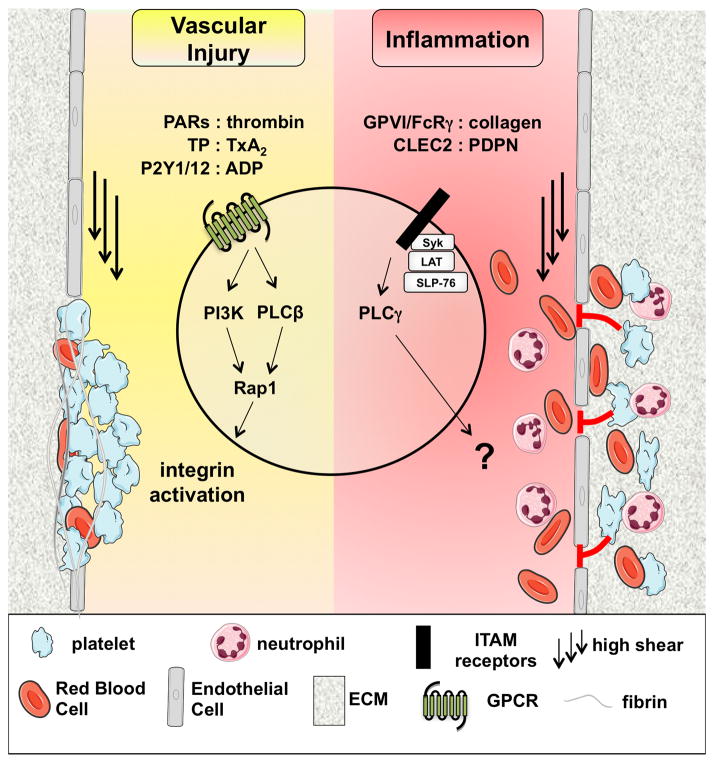
Figure 1. Platelet-dependent hemostasis after vascular injury and at sites of inflammation.
Schematic representation of important molecular mechanisms regulating platelet-dependent hemostasis. At sites of vascular injury, platelet activation and adhesion is strongly dependent on soluble agonists and their respective G protein-coupled receptors (GPCRs) expressed on the platelet surface. Engagement of GPCRs leads to the rapid activation of phospholipase (PL)Cβ2 and PI3 kinase, events that are critical for the activation of the small GTPase Rap1, affinity regulation in platelet integrins, and platelet aggregate formation. The contribution of immunoreceptor tyrosine-based activation motif (ITAM)-coupled receptors to platelet activation at sites of vascular injury is weak when compared to GPCRs. In contrast, hemostasis at sites of inflammation depends primarily on platelet ITAM signaling and is independent of major platelet adhesion receptors. These findings suggest a model where platelets get activated under low/no flow conditions in the extravascular space, leading to the release of soluble factors that secure vascular integrity. Both the signaling response downstream of PLCγ2 and the platelet-derived mediator(s) critical for vascular integrity in inflammation are currently unknown.
PAR: protease activated receptor; TxA2: thromboxane A2; TP: TxA2 receptor; FcRγ: Fc receptor γ-chain; CLEC2: C-type lectin 2; PDPN: podoplanin; PI3K: PI3 kinase; PLC: phospholipase C; ECM: extracellular matrix