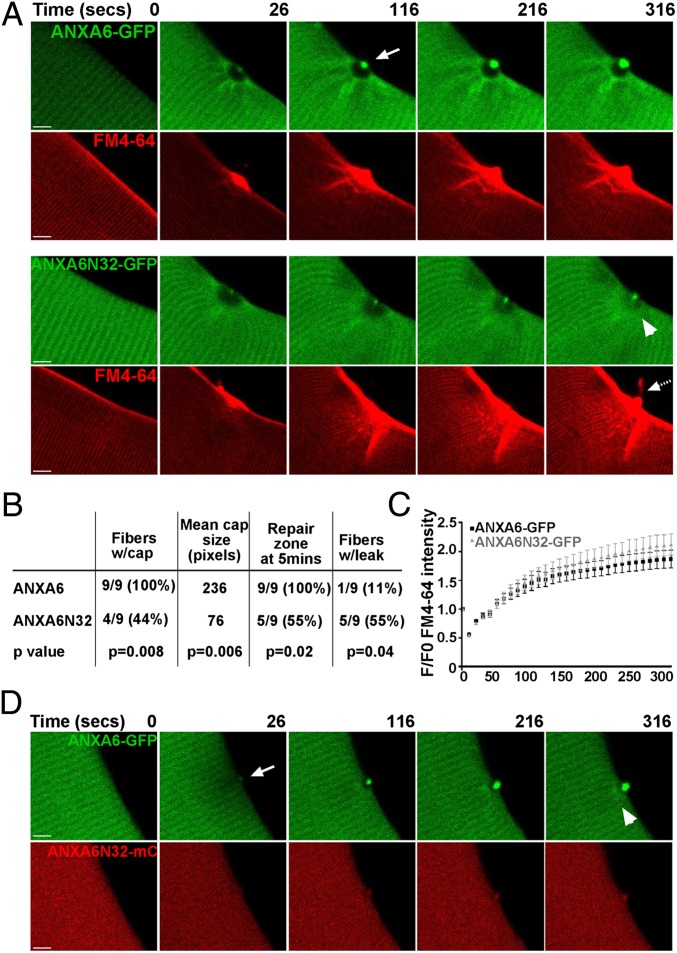
Fig. 4.
ANXA6N32 disrupts membrane resealing after membrane injury. WT129 myofibers were electroporated with ANXA6-GFP or ANXA6N32-GFP for live cell imaging after laser damage. (A) ANXA6-GFP (green) readily formed a distinct cap at the site of membrane disruption (arrow). This cap was visible by 26 s and persisted at 316 s after laser disruption. Concurrent imaging for FM4-64 showed that the cap forms over a vesicle-rich area devoid of annexin A6, referred to as the repair zone. In contrast, truncated annexin 6, ANXA6N32-GFP, formed a much smaller cap. The FM4-64 rich repair zone with ANXA6N32 was disorganized and hazy (compare GFP images at 316 s, arrowhead) and was frequently associated with leak of FM4-64 (dashed arrow). (Scale bar, 5 µm.) (B) Quantitation of laser experiments indicating that ANXA6N32 was associated with statistically smaller repair caps and leak. Full-length ANXA6-GFP formed a repair cap in 100% of damaged fibers. ANXA6N32-GFP formed a repair cap in only 44% of damaged fibers with a smaller mean size of the repair cap in those fibers that formed a cap (P < 0.006, n = 9). The repair zone of ANXA6-GFP persisted through 5 min in 100% of fibers, whereas the repair zone in ANXA6N32-GFP fibers persisted in only 55% of fibers. FM4-64 leak from the fiber occurred in 55% of ANXA6N32-GFP fibers at the site of damage, whereas only one ANXA6-GFP fiber (11%) showed FM4-64 efflux. (C) FM4-64 entry was quantified showing delayed repair with ANXA6N32 compared with ANXA6. (D) Expression of ANXA6-GFP along with ANXA6N32-mcherry (mC) demonstrated that ANXA6N32 disrupted the repair zone and cap formed by ANXA6, consistent with a dominant negative effect.