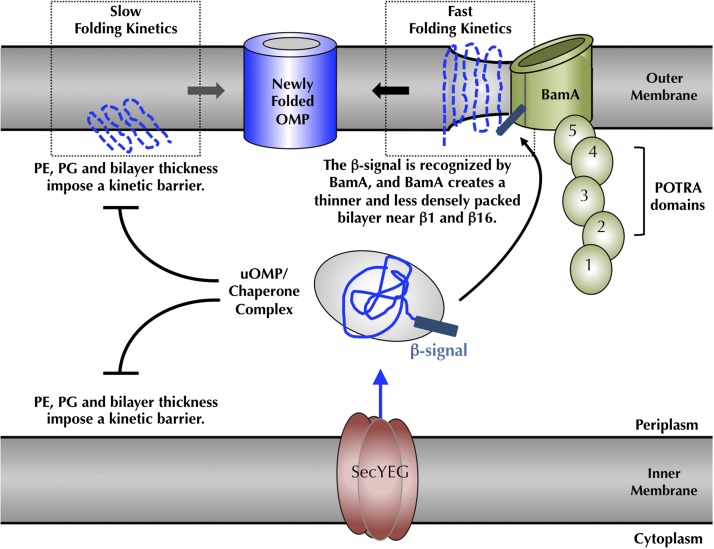
Fig. 5.
Cellular consequences of the lipid-induced kinetic folding barrier and model for BamA-catalyzed folding. Folding of bacterial OMPs into the outer membrane of E. coli is kinetically prevented by the bilayer thickness and a densely packed membrane surface, caused by PE and PG head groups (15, 37–39). At the outer membrane, BamA accelerates client OMP folding by the creation of local defects in and thinning of the membrane bilayer (32), which promote faster intrinsic folding kinetics (15). β-signals of client OMPs are recognized by BamA, which localizes clients to the site of the membrane defect. A high Km value of BamA for client OMPs together with favorable OMP folding free energies (49) drive the client OMPs to dissociate from BamA and move away into the membrane. This mechanistic view indicates how bacterial and mitochondrial OMPs can be recognized and folded by the eukaryotic BamA homologs (44, 45). POTRA, polypeptide-transport-associated.