Abstract
Background
Nasopharyngeal carcinoma (NPC) is a common malignancy in Southeast Asia, however, a full consensus has not yet been reached as to the value of comprehensive treatment for NPC. This study was designed to evaluate the epidemiological characteristics of NPC and their prognostic value, as well as the long-term efficacy of NPC treatment.
Patients and methods
A total of 248 patients, with different stages of NPC, were included in this study.
Results
The 5-year overall survival (OS) rates for patients in stages I, II, III and IV were 90.48%, 76.71%, 76.89% and 33.87%, respectively (P=0.000), while the respective 5-year progression-free survival (PFS) rates were 85.15%, 72.36%, 63.88% and 26.26% (P=0.000). The respective 5-year OS rates, according to stage, for the group that received radiotherapy combined with chemotherapy and for the group that received radiotherapy only were as follows: stages I and II, 81.67% and 79.59% (P=0.753); stage III, 79.91% and 70.38% (P=0.143); stage IV, 35.22% and 0% (P=0.000). The respective 5-year PFS rates in these groups were as follows: stages I and II, 75.83% and 74.98% (P=0.814); stage III, 74.08% and 42.25% (P=0.027); stage IV, 27.31% and 0% (P=0.000).
Conclusions
Clinical staging appears to be the most important prognostic factor for NPC. As the stage number increases, both the 5-year OS and PFS significantly decrease. Adding chemotherapy to radiotherapy was not advantageous for patients with stage I or II NPC, however the addition of chemotherapy to radiotherapy significantly improved OS and PFS in patients with stage IV NPC. The addition of chemotherapy improved PFS, but not OS in patients with stage III NPC.
Keywords: Nasopharyngeal carcinoma (NPC), treatment, retrospective analysis, Macao
Introduction
According to world cancer statistics, nasopharyngeal carcinoma (NPC) is rare, with an incidence rate in men of 0.6-2.1/100,000 (1). However, NPC is a much more common malignancy in Southeast Asia, especially in the southern coastal area of Mainland China and in Hong Kong, Macao and Taiwan. The annual incidence rate among the male population in Hong Kong is about 20/100,000 (2). The incidence of NPC gradually increases with age, peaking at 50-59 years of age, and then tends to decrease (2). Furthermore, the prognosis for those with NPC tends to worsen with age (3). There is a notable difference in the pathological types of NPC that occur within different regions. The keratinizing type of NPC (WHO Type I) mainly occurs in Western countries with overall low incidences of NPC. However, NPC tends to be of an undifferentiated, non-keratinizing subtype (WHO Type III) in South China and in Southeast Asian countries where the overall incidence of NPC is higher (4,5). Patient survival rates also differ depending on the different pathological type of NPC. Patients with Type III have significantly better survival rates compared to those with Type I (6,7).
Surgical resection is very difficult due to the fact that NPC is anatomically deep and occurs close to important neurovascular structures. Thus, the mainstay strategies for the treatment of NPC are radiotherapy-based, comprehensive therapies, including concurrent chemoradiotherapy, as well as induction or adjuvant chemotherapy and palliative chemotherapy following radiotherapy (8). Prognosis is affected by treatment approach, race, histological type, and disease stage (7,9,10). Many factors that impact the long-term survival of NPC patients have not yet been fully clarified (11-13). In the current study, the clinical data from NPC patients in the Macao region was retrospectively analyzed in order to clarify epidemiological characteristics, influencing factors and patient survival.
Patients and methods
Clinical data was collected from all histologically confirmed, new cases of NPC, which occurred between 2005 and 2009, at Macao Conde S. Januario General Hospital. Patients that had been previously diagnosed with NPC and treated, but who had relapsed during this period, were excluded. However, the study did include patients that had been diagnosed with NPC outside of Macao between 2005 and 2009, who then underwent subsequent treatment and follow-up at Conde S. Januario General Hospital.
Data collected included patient demographics, NPC stage, histological type, treatment modalities, treatment efficacy, and survival time. Patient demographic characteristics included gender, age, and marital status. Age data were divided into two groups: those younger than 50 years of age and those 50 years of age and older. The disease was restaged in accordance with the seventh edition of the American Joint Committee of Cancer (AJCC) TNM staging system (14) and the tumor pathological types were determined according to the WHO’s NPC classification (15).
The treatment modalities were radiotherapy alone, neoadjuvant chemotherapy followed by radiotherapy, concurrent chemoradiotherapy plus adjuvant chemotherapy, or palliative treatment. The agents used in neoadjuvant chemotherapy were cisplatin combined with 5-FU. The concurrent chemoradiotherapy and adjuvant chemotherapy treatments were the same as those used by Zhang et al. (16). Palliative treatment of advanced tumors included single-agent chemotherapy or combined chemotherapy and radiotherapy.
All patients in the two groups had received intensity modulated radiation therapy (IMRT) as reported by Ma et al. (17). The split-field technique, consisting of two lateral-opposed facial fields, was used during the course of radiation for patients with tumors confined to the nasopharynx, and for some cases an anterior field was also used if necessary. In cases of nasal or ethmoidal involvement, an additional anterior facial electron field was also involved. If the tumor remained in the primary site after 70 Gy was delivered, the total dose was boosted to 80 Gy with the cone-down technique. In patients with skull base involvement and intracranial extension, boost doses of 10 to 14 Gy in 5 to 7 fractions were given to the corresponding positions. The total dosage for all palpable residual tumors could be boosted to 70 Gy with an electron field (9 to 12 Mev) at the 90% isodose level when all the external radiotherapy plans were completed.
Patients were examined prior to treatment and during the follow-up period after treatment. Examinations included a complete medical history and physical examination, a craniofacial examination (including dental and cranial nerve exams), nasopharyngofiberscopy, a complete blood count, serum biochemistry, a chest X-ray, and a CT or MRI examination of the nasopharynx, skull base and any suspicious metastatic sites, including the paranasal sinuses. Treatment efficacy was evaluated using WHO criteria (18). After treatment, the patients were asked to return to the clinic once every three months, for two years and then every six months until relapse or death. The follow-up period was defined as the period from the date of diagnosis until death or until the last follow-up time. Patients with disease recurrence, progression, and those that were lost to follow-up were considered to have died on the day of their last follow-up. Local recurrence was confirmed by examination of the nasopharynx, head and neck and was verified by needle aspiration biopsy or MRI. Distant metastases were identified by clinical symptoms, physical examination, or bone scans and verified by CT or MRI scan(s).
Prognostic factors for NPC patients were determined by analyzing the associations between patient survival and the following: age, gender, disease-stage, NPC histological type, treatment modalities and primary therapeutic effects. Overall survival (OS) was defined as the time from diagnosis to the time of death from any cause. The cut-off time for patients who survived was defined as the time of last visit. Progression-free survival (PFS) referred to the time from the start of treatment until recurrence, disease progression or death from any cause. The cut-off time for the cases without disease progression was defined as the time of last visit. SPSS 13.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. The survival curves were analyzed using the Kaplan-Meier method and the survival curves of different groups of patients were compared using a log-rank test. The COX proportional hazard model was used for multivariate analysis of the prognostic factors, including the patient, tumor and treatment modalities.
Results
A total of 248 newly diagnosed cases of NPC were treated in Conde S. Januario General Hospital between January 1, 2005 and December 31, 2009. The age of disease onset was similar to normal distribution, with a median age of 49.0 years (Table 1).
Table 1. Characteristics of patients.
| Characteristics | Number | % |
| Age (years) | ||
| Range | 19-100 | |
| Median | 49.00 | |
| Male:Female | 184:64 | |
| Histology | ||
| WHO Type I | 14 | 5.65 |
| WHO Type II | 8 | 3.22 |
| WHO Type III | 226 | 91.13 |
| Stage (AJCC 2010) | ||
| Stage I | 21 | 8.47 |
| Stage II | 79 | 31.85 |
| Stage III | 96 | 38.72 |
| Stage IV | 52 | 20.97 |
| T classification | ||
| T1 | 42 | 16.94 |
| T2 | 117 | 47.18 |
| T3 | 63 | 25.40 |
| T4 | 26 | 10.48 |
| N classification | ||
| N0 | 65 | 26.21 |
| N1 | 85 | 34.27 |
| N2 | 73 | 29.44 |
| N3 | 25 | 10.08 |
| M classification | ||
| M0 | 233 | 93.95 |
| M1 | 15 | 6.05 |
| Primary treatment | ||
| RT | 118 | 47.58 |
| RT + CT | 130 | 52.42 |
AJCC, American Joint Committee on Cancer; RT, radiotherapy; CT, chemotherapy.
The median follow-up time was 47.5 months (range, 1-109 months). The 5-year survival rate for all patients that underwent follow-up was 68.70%, however, the median survival (5-year) was not reached and could not be calculated. The 5-year survival rates for patients with stages I, II, III, and IV NPC were 90.48%, 76.71%, 76.89% and 33.87%, respectively. There was a significant difference in the survival curves among patients in different clinical stages (P=0.000) (Figure 1). The prognostic significances of age, gender, T stage, M stage, primary treatment modality, and therapeutic effect were evaluated by univariate analysis (Table 2). Favorable prognostic indicators for relatively longer OS included being less than 50 years of age, being female, having an earlier T stage, the absence of distant metastases, having had treatment with radiotherapy alone, and achieving remission after first-line treatment (P<0.05). Age, gender, T stage, M stage, primary treatment modality and initial therapeutic effect were all independent prognostic factors for OS, as indicated by multivariate COX regression analysis (Table 3).
Figure 1.
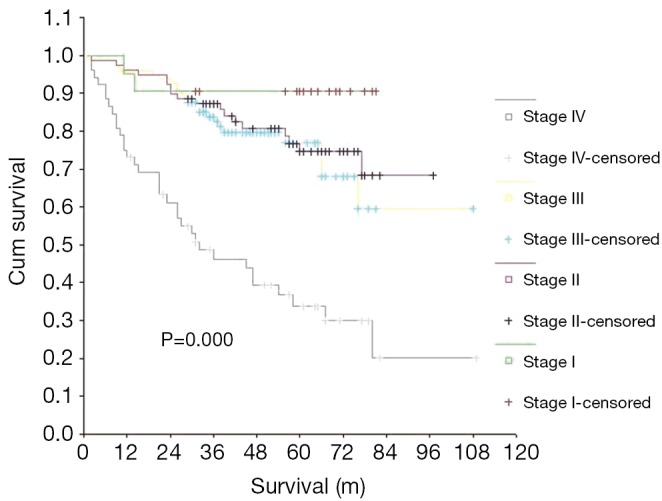
Comparison of OS among the AJCC stages. OS, overall survival; AJCC, American Joint Committee on Cancer.
Table 2. Univariate analysis of prognostic factors for OS and PFS.
| Factors | n | 5-year OS rate (%) | P value | 5-year PFS rate (%) | P value |
|---|---|---|---|---|---|
| Age group | |||||
| <50 | 131 | 76.07 | 0.019 | 64.92 | 0.061 |
| 50 and above | 117 | 60.34 | 56.49 | ||
| Gender | |||||
| Male | 184 | 62.85 | 0.004 | 54.96 | 0.003 |
| Female | 64 | 85.73 | 78.39 | ||
| Stage (AJCC 2010) | |||||
| Stage I | 21 | 90.48 | 0.000 | 85.15 | 0.000 |
| Stage II | 79 | 76.71 | 72.36 | ||
| Stage III | 96 | 76.89 | 63.88 | ||
| Stage IV | 52 | 33.87 | 26.26 | ||
| T classification | |||||
| T1 | 42 | 86.03 | 0.000 | 79.75 | 0.000 |
| T2 | 117 | 70.31 | 64.13 | ||
| T3 | 63 | 67.11 | 54.51 | ||
| T4 | 26 | 32.11 | 27.34 | ||
| M classification | |||||
| M0 | 233 | 72.75 | 0.000 | 64.97 | 0.000 |
| M1 | 15 | 6.67 | 0.00 | ||
| Primary treatment | |||||
| RT | 118 | 75.51 | 0.026 | 67.08 | 0.038 |
| RT + CT | 130 | 61.45 | 55.58 | ||
| Treatment effect | |||||
| No evaluate | 5 | 0.00 | 0.000 | – | 0.000 |
| CR | 218 | 75.50 | 67.97 | ||
| PR | 14 | 31.75 | 21.43 | ||
| NC | 3 | 0.00 | 0.00 | ||
| PD | 8 | 12.50 | 0.00 |
AJCC, American Joint Committee on Cancer; RT, radiotherapy; CT, chemotherapy, CR, complete response; PR, partial response, NC, no change; PD, progression disease; OS, overall survival; PFS, progression-free survival.
Table 3. Multivariate analysis of prognostic factors for OS and PFS.
| Variables | No. of patients | OS |
PFS |
|||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |||
| Age group | ||||||||
| <50 | 131 | 2.40 | 1.49-3.86 | 0.000 | 1.90 | 1.28-2.99 | 0.002 | |
| 50 and above | 117 | |||||||
| Gender | ||||||||
| Male | 184 | 0.35 | 0.18-0.70 | 0.003 | 0.41 | 0.23-0.73 | 0.002 | |
| Female | 64 | |||||||
| Histology | ||||||||
| WHO Type I | 14 | 0.75 | 0.47-1.19 | 0.219 | 1.06 | 0.73-1.54 | 0.766 | |
| WHO Type II | 8 | |||||||
| WHO Type III | 226 | |||||||
| Stage (AJCC 2010) | ||||||||
| Stage I | 21 | 3.06 | 1.96-4.78 | 0.000 | 2.95 | 2.00-4.36 | 0.000 | |
| Stage II | 79 | |||||||
| Stage III | 96 | |||||||
| Stage IV | 52 | |||||||
| T classification | ||||||||
| T1 | 42 | 1.97 | 1.43-2.70 | 0.000 | 1.78 | 1.35-2.36 | 0.000 | |
| T2 | 117 | |||||||
| T3 | 63 | |||||||
| T4 | 26 | |||||||
| N classification | ||||||||
| N0 | 65 | 1.38 | 0.91-2.10 | 0.132 | 1.21 | 0.81-1.80 | 0.347 | |
| N1 | 85 | |||||||
| N2 | 73 | |||||||
| N3 | 25 | |||||||
| M classification | ||||||||
| M0 | 233 | 4.63 | 1.38-15.59 | 0.013 | 3.75 | 1.28-10.96 | 0.016 | |
| M1 | 15 | |||||||
| Primary treatment | ||||||||
| RT | 118 | 0.32 | 0.16-0.63 | 0.001 | 0.39 | 0.22-0.70 | 0.002 | |
| RT + CT | 130 | |||||||
| Treatment effect | ||||||||
| No evaluate | 5 | 1.70 | 1.26-2.31 | 0.001 | 2.18 | 1.63-2.92 | 0.000 | |
| CR | 218 | |||||||
| PR | 14 | |||||||
| NC | 3 | |||||||
| PD | 8 | |||||||
AJCC, American Joint Committee on Cancer; RT, radiotherapy; CT, chemotherapy; CR, complete response; PR, partial response; NC, no change; PD, progression disease; OS, overall survival; PFS, progression-free survival; CI, confidence interval.
The 5-year PFS rate was 61.04% for all patients. The PFS rates for patients with stages I, II, III, and IV NPC were 85.15%, 72.36%, 63.88% and 26.26%, respectively. Just like the median survival, the median PFS (5-year) was not reached either. Clinical stage was found to be a significant prognostic indicator for PFS (P=0.000) (Figure 2). Univariate analysis showed that gender, T stage, M stage, primary treatment modality, and treatment efficacy were all prognostic factors for PFS in patients (Table 2). Favorable prognostic indicators for relatively long PFS included being a woman, having an earlier T stage, having no distant metastases, having had radiotherapy alone and achieving remission after primary treatment (P<0.05). Age, gender, T stage, M stage, the primary treatment modality, and the efficacy of first-line therapy were all independent prognostic factors for PFS, as indicated by multivariate analysis (Table 3).
Figure 2.
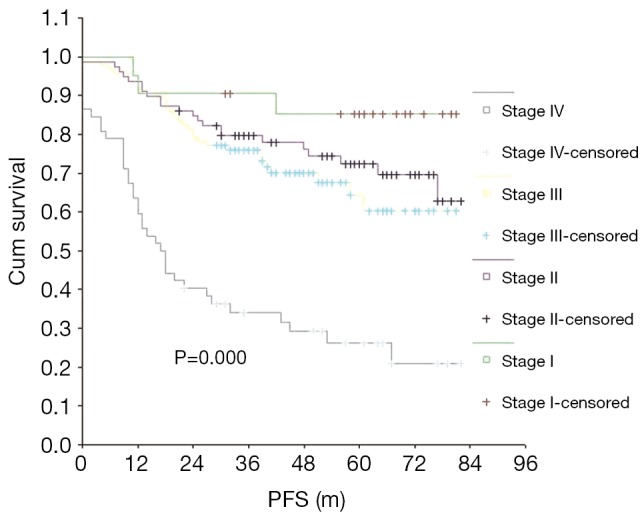
Comparison of PFS among the AJCC stages. PFS, progression-free survival; AJCC, American Joint Committee on Cancer.
The 5-year OS and PFS rates were significantly better in the radiotherapy-only group compared with the group that received a combination of radiotherapy and chemotherapy. The effect of treatment modality on long-term survival was further examined via subgroup analysis of the demographic characteristics, pathological type and stage of NPC in these two patient groups (Table 4). The group treated with a combination of chemotherapy and radiotherapy was at a significantly later stage of NPC, on average, than the group that was treated with radiotherapy alone, indicating that the patients were not randomly assigned to each group and that more late-stage patients received radiotherapy combined with chemotherapy. Long-term survival was further analyzed according to different subgroups excluding interference due to unbalanced stage distribution between the two groups (Table 5). When comparing the treatment modalities, there was no significant difference in the 5-year OS and PFS of patients with stages I and II NPC (P=0.753 and P=0.814, respectively) (Figure 3). The 5-year PFS in stage III patients treated with a combination of radiotherapy and chemotherapy was significantly better than stage III patients that were treated with radiotherapy alone (P=0.027), however, there was no significant difference in the 5-year OS of stage III patients when comparing these treatment modalities (P=0.143) (Figure 4). The 5-year OS and PFS were both significantly better for stage IV patients that received combined chemotherapy and radiotherapy compared with stage IV patients that were treated with radiotherapy alone (P=0.000 and P=0.000) (Figure 5).
Table 4. Characteristics of patients of different primary treatment modalities.
| Factors | N=248 | RT (n=118) | RT + CT (n=130) | P value |
|---|---|---|---|---|
| Age group | ||||
| <50 | 131 | 59 | 72 | 0.396 |
| 50 and above | 117 | 59 | 58 | |
| Gender | ||||
| Male | 184 | 84 | 100 | 0.302 |
| Female | 64 | 34 | 30 | |
| Histology | ||||
| WHO Type I | 14 | 7 | 7 | 0.674 |
| WHO Type II | 8 | 5 | 3 | |
| WHO Type III | 226 | 106 | 120 | |
| Stage (AJCC 2010) | ||||
| Stage I | 21 | 21 | 0 | 0.000 |
| Stage II | 79 | 64 | 15 | |
| Stage III | 96 | 31 | 65 | |
| Stage IV | 52 | 2 | 50 | |
| T classification | ||||
| T1 | 42 | 36 | 6 | 0.000 |
| T2 | 117 | 60 | 57 | |
| T3 | 63 | 15 | 44 | |
| T4 | 26 | 3 | 23 | |
| N classification | ||||
| N0 | 65 | 54 | 13 | 0.000 |
| N1 | 85 | 48 | 37 | |
| N2 | 73 | 15 | 58 | |
| N3 | 25 | 1 | 24 | |
| M classification | ||||
| M0 | 233 | 117 | 116 | 0.001 |
| M1 | 15 | 1 | 14 | |
AJCC, American Joint Committee on Cancer; RT, radiotherapy; CT, Chemotherapy.
Table 5. Five-year OS and PFS rates of different stages and treatment modalities.
| Variables | No. of patients (n=248) | OS rate (%) |
PFS rate (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| RT (n=118) | RT + CT (n=130) | P value | RT (n=118) | RT + CT (n=130) | P value | |||
| Stage (AJCC2010) | ||||||||
| Stage I | 100 | 79.59 | 81.67 | 0.036 | 74.98 | 75.83 | 0.012 | |
| Stage II | 96 | 70.38 | 79.91 | 42.25 | 74.08 | |||
| Stage III | 52 | 0 | 35.22 | 0 | 27.31 | |||
| T classification | ||||||||
| T1 | 42 | 89.67 | 66.67 | 0.953 | 88.21 | 25.00 | 0.778 | |
| T2 | 117 | 76.05 | 63.10 | 68.79 | 58.97 | |||
| T3 | 63 | 57.89 | 70.18 | 30.06 | 67.88 | |||
| T4 | 26 | 0 | 36.44 | 0 | 30.91 | |||
| N classification | ||||||||
| N0 | 65 | 90.22 | 63.64 | 0.712 | 77.91 | 54.55 | 0.528 | |
| N1 | 85 | 61.21 | 65.93 | 58.53 | 60.81 | |||
| N2 | 73 | 65.19 | 69.84 | 58.33 | 63.26 | |||
| N3 | 25 | 100.00 | 40.12 | 100.00 | 32.41 | |||
| M classification | ||||||||
| M0 | 233 | 76.15 | 68.09 | 0.447 | 67.65 | 62.30 | 0.417 | |
| M1 | 15 | 0 | 7.14 | 0 | – | |||
RT, radiotherapy; CT, chemotherapy; AJCC, American Joint Committee on Cancer; OS, overall survival; PFS, progression-free survival.
Figure 3.
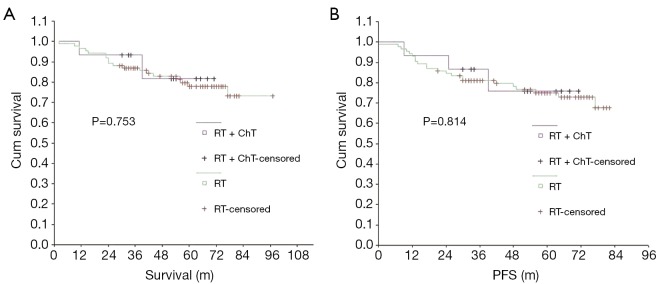
Comparison of OS (A) and PFS (B) between the treatment methods in stage I-II. OS, overall survival; PFS, progression-free survival.
Figure 4.
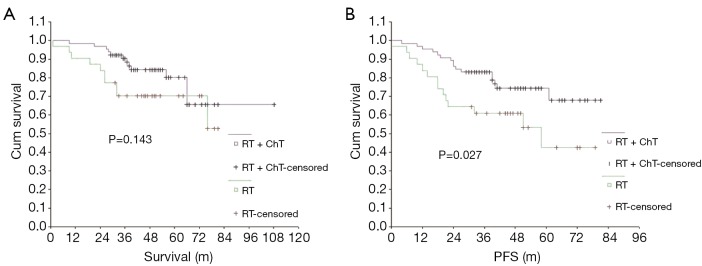
Comparison of OS (A) and PFS (B) between the treatment methods in stage III. OS, overall survival; PFS, progression-free survival.
Figure 5.
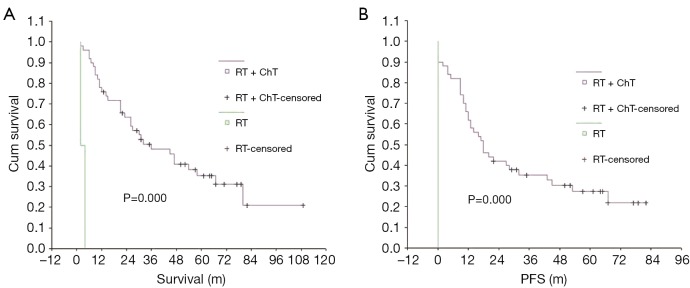
Comparison of OS (A) and PFS (B) between the treatment methods in stage IV. OS, overall survival; PFS, progression-free survival.
Discussion
In the current study, the five-year survival rate in patients older than 50 years of age was significantly lower than in patients younger than 50 years of age (P=0.019), however, there was no significant difference in 5-year PFS between the two age groups (P=0.061), indicating that the prognosis for OS in older patients is worse than in younger patients. Age was also found to be an independent prognostic factor affecting the long-term survival of patients with NPC. Similarly, a retrospective study done in Malaysia reported that the risk of death within five years for NPC patients older than 70 years of age was 3.18 times that of patients younger than 50 years of age (19).
Existing epidemiological data demonstrate that gender is a major factor in the incidence of NPC. The occurrence of NPC in males is 2-3 times that in females; this is similar to the results found in the current study (20,21). Previous studies have reported slightly better, though not significant, long-term survival rates in women with NPC than in men (19). However, in one Japanese retrospective study a significantly higher 5-year survival rate was reported for female patients compared to male patients (P=0.032), though multivariate analysis showed that gender was not an independent prognostic factor for survival in this study (20). In the current study, the 5-year OS rate for male and female patients was 62.85% and 85.73% (P=0.004) and the 5-year PFS rate was 54.96% and 78.39% (P=0.003), respectively, suggesting that gender was an independent prognostic factor affecting OS and PFS.
Some studies have demonstrated that patients with WHO Type III NPC are more sensitive to radiotherapy, and survive longer, than those with Type I (22). However, there is not yet enough evidence that supports the idea that patients with different pathological types of NPC require different treatment modalities (5,23). In the current study, patients with WHO Type III NPC accounted for 91.13% of cases, while those with Types I and II accounted for only 8.87% of cases. No statistically significant difference found in OS (P=0.219) or PFS (P=0.766) among the different pathological types in the current study, though this is contrary to previously published results (19,20). A retrospective study conducted in Malaysia showed that the risk of death was 1.97 times greater in patients with WHO Types I and II NPC than in patients with type III (19). Similarly, in a retrospective Japanese study, patients with the nonkeratinizing type of NPC (WHO Types III and II) were found to have higher 5-year OS and PFS rates than those with the keratinizing type (WHO Type I) (20). Only one study, conducted in Brazil, reported results similar to the current study and concluded that there was no significant difference in the 5-year disease-specific survival rates among different histological types of NPC (24).
Many studies have confirmed a clear association between long-term survival and NPC clinical stage (19,20,22,24,25). Results of the current study also demonstrated that, as the disease stage increased, the 5-year OS and PFS rates gradually and significantly decreased (P=0.000). Multivariate analysis established AJCC staging as an independent prognostic indicator for OS and PFS. Further analysis demonstrated that tumor size (T) and distant metastases (M) are decisive factors for OS and PFS, but that lymph node metastasis (N) staging has no independent prognostic significance for OS or PFS. The 5-year OS and PFS rates in patients with stage T1 were 2.68 and 2.92 times that of patients with T4, respectively. Similarly, in patients with M1, the risk of death was 4.63 times higher and the risk of recurrence and progression was 3.75 times higher than in patients with M0.
The efficacy of primary treatment was also found to be an independent prognostic factor affecting long-term survival. Among all 248 patients, the complete remission (CR) rate was 87.90% and the partial remission (PR) rate was 5.65%. The 5-year OS and PFS rates were significantly higher in patients with CR and PR than in patients without remission (P=0.000 and P=0.000, respectively). Therefore, the initial treatment modality and its therapeutic efficacy are the major factors affecting the prognosis of patients in all stages of NPC. All measures should be taken to achieve CR or PR during primary treatment as this will improve the long-term survival of patients in all stages of NPC.
NPC is sensitive to radiotherapy and chemotherapy (26,27). Since surgical resection is difficult and the efficacy is poor, the primary treatment for NPC is radiotherapy and chemotherapy is used as an adjuvant option. Surgical resection is limited to cases in which there is residual tumor or can be used as a salvage therapy in cases of local recurrences. Although NPC is relatively sensitive to radiotherapy, the long-term survival for patients with advanced NPC is not ideal (23,28,29). According to the literature, the five-year survival rate for patients with stage IV NPC, who received radiotherapy only, is between 28% and 35% (30,31). Therefore, appropriate addition of chemotherapy is necessary to improve long-term survival in these patients.
Many studies have shown that adding chemotherapy to radiotherapy can improve treatment efficacy and prolong OS in patients with intermediate or advanced NPC, though not all studies have had positive results (13,24,32-35). Phua et al. found no difference in the prognosis among patients with different stages of NPC whether chemotherapy was added or not (19). A retrospective analysis by Chua et al. found that radiotherapy combined with induction chemotherapy resulted in only a mild improvement in PFS and in the relapse rate and no improvement in OS when compared with radiotherapy alone (13). A phase III clinical trial conducted in patients with locally advanced NPC in China showed that adding adjuvant chemotherapy did not result in improved OS or relapse-free survival when compared with using concurrent chemoradiotherapy (36). Chen et al. reported that, in a randomized phase III trial, the 5-year survival in 230 cases of stage II NPC was significantly better in the group treated with combined radiotherapy and chemotherapy compared to the group treated with radiotherapy alone (37). The prolonged survival in the combined group was mainly attributed to a lower rate of distant metastases, however, restaging these patients according to the latest TNM classification system [2010] revealed that a considerable portion of the patients should have been categorized as stage III (14).
The group that received combined chemotherapy and radiotherapy was compared with the group that received radiotherapy only to determine the effect of adding chemotherapy on patient survival in our study. The 5-year OS and PFS rates in the combined chemotherapy and radiotherapy group were significantly lower than in the radiotherapy only group (P=0.026 and P=0.038, respectively). However, according to AJCC stage-based subgroup analysis, there was no difference between the two groups in the 5-year OS rate of patients with stages I, II and III NPC, although the 5-year OS rate of patients with stage IV NPC was significantly higher in the chemotherapy combined with radiotherapy group than in the radiotherapy only group. For patients with stage I or II NPC, the 5-year PFS rate was not significantly different in the combined group vs. the radiotherapy only group (P=0.814). Conversely, for patients with stage III or IV NPC, the rate of 5-year PFS was significantly higher in the combined group than in the radiotherapy only group (P=0.027 and P=0.000, respectively).
Patients with stages I or II NPC will likely not benefit from the addition of chemotherapy, in terms of long-term survival and PFS. However, for patients with stage III NPC, adding chemotherapy can improve PFS to a certain degree though it may not improve OS and in patients with stage IV NPC, the addition of chemotherapy can significantly prolong both OS and PFS. A random trial from endemic regions of China also showed the addition of concurrent and adjuvant chemotherapy to RT provides survival benefits to patients with stage III through IVB NPC (35).
Conclusions
Therefore, in clinical practice, it is recommended that chemotherapy be added to radiotherapy for patients with stage IV NPC. Treatment modalities may include induction chemotherapy, concurrent chemoradiotherapy, and adjuvant or palliative chemotherapy treatment after radiotherapy. In patients with stage III NPC, the treatment should be based on the individual. Chemotherapy may be considered for patients that are otherwise in good general health or for patients that have a relatively advanced stage of NPC. In patients with mid and early stages of NPC, such as stage II or lower, chemotherapy is not recommended.
However, this study was a non-randomized, retrospective analysis and the clinical stage between the two groups was unbalanced, which may have led to bias in our results. Thus, a randomized phase III study is necessary to further verify our conclusions.
Acknowledgements
We thank David Lopes, Pinho Pereira, Jianfeng Zhou from the Department of Haemato-oncology, Conde S. Januario General Hospital, Macao SAR, for their assistance in data collection.
Disclosure: The authors declare no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90 [DOI] [PubMed] [Google Scholar]
- 2.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 2006;15:1765-77 [DOI] [PubMed] [Google Scholar]
- 3.Daoud J, Toumi N, Bouaziz M, et al. Nasopharyngeal carcinoma in childhood and adolescence: analysis of a series of 32 patients treated with combined chemotherapy and radiotherapy. Eur J Cancer 2003;39:2349-54 [DOI] [PubMed] [Google Scholar]
- 4.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet 2005;365:2041-54 [DOI] [PubMed] [Google Scholar]
- 5.Sun LM, Li CI, Huang EY, et al. Survival differences by race in nasopharyngeal carcinoma. Am J Epidemiol 2007;165:271-8 [DOI] [PubMed] [Google Scholar]
- 6.Marks JE, Philips JL, Menck HR. The National Cancer Data Base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer 1998;83:582-8 [DOI] [PubMed] [Google Scholar]
- 7.Nelson V, Rademaker A, Kaklamani V.Paradigm of polyendocrine therapy in endocrine responsive breast cancer: the role of fulvestrant. Chin Clin Oncol 2013;2:10. [DOI] [PubMed] [Google Scholar]
- 8.Lin A, Swisher-McClure S, Millar LB, et al. Proton therapy for head and neck cancer: current applications and future directions. Transl Cancer Res 2012;1:255-63 [Google Scholar]
- 9.Li X, Xiao S, Li Y, et al. Clinical antiangiogenic effect of recombinant adenovirus-p53 combined with hyperthermia for advanced cancer. Chin J Cancer Res 2013;25:749-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chedid HM, Franzi SA, Dedivitis RA, et al. Assessment of clinical and therapeutic factors in patients with nasopharyngeal undifferentiated carcinoma. Braz J Otorhinolaryngol 2008;74:566-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chua DT, Ma J, Sham JS, et al. Improvement of survival after addition of induction chemotherapy to radiotherapy in patients with early-stage nasopharyngeal carcinoma: Subgroup analysis of two Phase III trials. Int J Radiat Oncol Biol Phys 2006;65:1300-6 [DOI] [PubMed] [Google Scholar]
- 12.Cheng SH, Tsai SY, Yen KL, et al. Concomitant radiotherapy and chemotherapy for early-stage nasopharyngeal carcinoma. J Clin Oncol 2000;18:2040-5 [DOI] [PubMed] [Google Scholar]
- 13.Chua DT, Ma J, Sham JS, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol 2005;23:1118-24 [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, et al. eds. AJCC (American Joint Committee on Cancer) cancer staging manual. 7th ed. New York: Springer, 2010:41-56. [Google Scholar]
- 15.Barnes L, Eveson JW, Reichart P, et al. eds. World Health Organization classification of tumours. Pathology and genetics of head and neck tumors. Lyon: IARC Press, 2005. [Google Scholar]
- 16.Zhang W, Dou H, Lam C, et al. Concurrent chemoradiotherapy with or without adjuvant chemotherapy in intermediate and locoregionally advanced nasopharyngeal carcinoma. Tumour Biol 2013;34:1729-36 [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Mai HQ, Hong MH, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol 2001;19:1350-7 [DOI] [PubMed] [Google Scholar]
- 18.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981;47:207-14 [DOI] [PubMed] [Google Scholar]
- 19.Phua CE, Tan BS, Yong TK, et al. Retrospective analysis of results of treatment for nasopharyngeal carcinoma in Penang General Hospital from 2001-2005. Asian Pac J Cancer Prev 2011;12:3197-200 [PubMed] [Google Scholar]
- 20.Kawashima M, Fuwa N, Myojin N, et al. A multi-institutional survey of the effectiveness of chemotherapy combined with radiotherapy for patients with nasopharyngeal carcinoma. Jpn J Clin Oncol 2004;34:569-83 [DOI] [PubMed] [Google Scholar]
- 21.Hui EP, Leung SF, Au JS, et al. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer 2004;101:300-6 [DOI] [PubMed] [Google Scholar]
- 22.Goto Y, Kodaira T, Fuwa N, et al. Alternating chemoradiotherapy in patients with nasopharyngeal cancer: prognostic factors and proposal for individualization of therapy. J Radiat Res 2013;54:98-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geara FB, Sanguineti G, Tucker SL, et al. Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of distant metastasis and survival. Radiother Oncol 1997;43:53-61 [DOI] [PubMed] [Google Scholar]
- 24.Farias TP, Dias FL, Lima RA, et al. Prognostic factors and outcome for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2003;129:794-9 [DOI] [PubMed] [Google Scholar]
- 25.Lee AW, Sze WM, Au JS, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2005;61:1107-16 [DOI] [PubMed] [Google Scholar]
- 26.al-Sarraf M, McLaughlin PW. Nasopharyngeal carcinoma: choice of treatment. Int J Radiat Oncol Biol Phys 1995;33:761-3 [DOI] [PubMed] [Google Scholar]
- 27.Schantz SP, Harrison LB, Forastiere AA. Tumors of the nasal cavity and paranasal sinuses, nasopharynx, oral cavity and oropharynx. In: DeVita VT, Hellman S, Rosenberg SA. eds. Cancer: Principles and Practice of Oncology. 5th ed. Philadelphia, Pa: Lippincott-Raven, 1997:741-801. [Google Scholar]
- 28.Teo P, Yu P, Lee WY, et al. Significant prognosticators after primary radiotherapy in 903 nondisseminated nasopharyngeal carcinoma evaluated by computer tomography. Int J Radiat Oncol Biol Phys 1996;36:291-304 [DOI] [PubMed] [Google Scholar]
- 29.Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys 1992;23:261-70 [DOI] [PubMed] [Google Scholar]
- 30.Heng DM, Wee J, Fong KW, et al. Prognostic factors in 677 patients in Singapore with nondisseminated nasopharyngeal carcinoma. Cancer 1999;86:1912-20 [PubMed] [Google Scholar]
- 31.Marcial VA, Hanley JA, Chang C, et al. Split-course radiationtherapy of carcinoma of the nasopharynx: results of a national collaborative clinical trial of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:409-14 [DOI] [PubMed] [Google Scholar]
- 32.Park KH, Kim JS, Park Y, et al. Concurrent chemoradiation followed by adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma in Korea. Cancer Chemother Pharmacol 2010;66:643-51 [DOI] [PubMed] [Google Scholar]
- 33.Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with Radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol 2002;20:2038-44 [DOI] [PubMed] [Google Scholar]
- 34.Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2010;102:1188-98 [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Sun Y, Liang SB, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer 2013;119:2230-8 [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012;13:163-71 [DOI] [PubMed] [Google Scholar]
- 37.Chen QY, Wen YF, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst 2011;103:1761-70 [DOI] [PubMed] [Google Scholar]


