Figure 3.
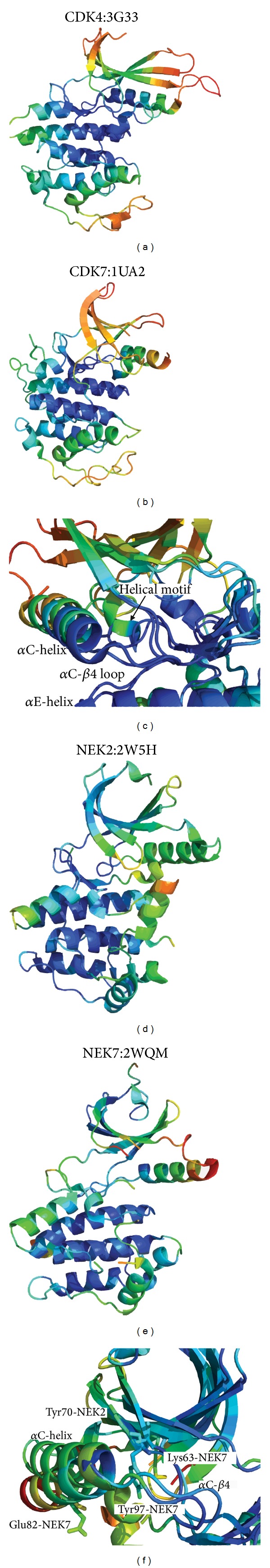
Functional dynamics and protein mobility maps of the Cdk and Nek subfamilies of kinases. Conformational mobility maps of the of the Cdk (Cdk4, Cdk7) (a, b, c) and Nek subfamilies (Nek2, Nek7) of kinases (d, e, f) were computed using MD simulations of the inactive crystal structures. Structural distribution of the protein kinase mobility is averaged over the two lowest frequency modes. A surface-based protein representation is employed and colored (blue-to-red) according to the protein residue motilities (from more rigid-blue regions to more flexible-red regions). A close-up of the regulatory hinge site formed by the αE-helix, αC-β4 loop, and the αC-helix is shown. The characteristic autoinhibitory interactions are formed between a small helical motif within the activation loop and the αC-helix that prevents access to the active enzyme form. The conserved functional residues (Tyr-70 in Nek2 and Tyr-97 in Nek7 shown in sticks on (f)) are projected from the β4 strand of the N-terminal right down into the hinge site between the αC-β4-loop and the αC-helix. These residues are implicated in regulatory interactions of Nek kinases with binding partners.
