Abstract
Clinical studies have demonstrated the effectiveness of hyperthermia as an adjuvant for chemotherapy and radiotherapy. However, significant clinical challenges have been encountered, such as a broader spectrum of toxicity, lack of patient tolerance, temperature control and significant invasiveness. Hyperthermia induced by magnetic nanoparticles in high-frequency oscillating magnetic fields, commonly termed magnetic fluid hyperthermia, is a promising form of heat delivery in which thermal energy is supplied at the nanoscale to the tumor. This review discusses the mechanisms of heat dissipation of iron oxide-based magnetic nanoparticles, current methods and challenges to deliver heat in the clinic, and the current work related to the use of magnetic nanoparticles for the thermal-chemopotentiation of therapeutic drugs.
Keywords: magnetic fluid hyperthermia, magnetic nanoparticle, synergy, thermal potentiation
Magnetic nanoparticles & magnetic fluid hyperthermia
Magnetic nanoparticles typically used in biomedical applications consist of small (5–20 nm diameter) inorganic crystals of a magnetic material that is either coated with an organic layer in a core–shell configuration or embedded in an organic matrix in the form of a multicore–shell configuration. The inorganic crystals may correspond to a variety of materials known to possess either superparamagnetic, ferrimagnetic, or ferromagnetic behavior; however, the most prevalent materials are the so-called iron oxides, magnetite [1] and maghemite [1], owing to their apparent lack of toxicity and biocompatibility. In this case, the small size of the inorganic crystals results in the formation of single magnetic domain particles with superparamagnetic behavior [2]. The organic shell is typically added to confer colloidal stability [3] in aqueous and biological fluids, and as a means to add functionality, such as fluorescent tags and targeting ligands.
A wide variety of organic shells have been investigated, including polysaccharides (e.g., dextran [4–10], carboxymethyl dextran [11,12] and chitosan [5,13–16]) and biocompatible polymers (e.g., PEG [5,17–21]). In some cases, thin coatings, such as citrates [22–25] and dimercaptosuccinic acid [23,26–28], have also been used. Fluorescent tags are typically added to assist investigations of the tissue, cellular and subcellular localization of nanoparticles, using for example confocal laser scanning fluorescence microscopy. On the other hand, targeting ligands are used to enhance selective uptake of nanoparticles by tissues and cells, thereby enhancing an imaging or therapeutic outcome while minimizing side effects in nonintended tissues. Some examples of targeting ligands used with magnetic nanoparticles include simple biochemicals (e.g., folic acid) [5,17,18,29–31], antibodies [28,32,33], antibody fragments [34,35], receptor ligands [36,37] and aptamers [38–41].
The growing interest in magnetic nanoparticles for biomedical applications stems, in part, from their ability to respond to applied magnetic fields by translation (in magnetic field gradients), physical particle rotation (in alternating and rotating fields) or internal dipole rotation (in alternating and rotating magnetic fields). As a result, there is local conversion of magnetic field energy into either mechanical forces and/or torques exerted on biological structures, or thermal energy. Some of these properties, such as the possibility of externally influencing their motion and the possibility of applying mechanical forces/torques on biological structures appear to be unique to magnetic nanoparticles.
Iron oxide nanoparticles enjoy a privileged position relative to other nanoparticle platforms being developed for biomedical applications in terms of their biocompatibility. Iron oxides have been used as part of iron supplementation regimens [42] and dextran-coated iron oxide nanoparticles gained US FDA approval for commercial application as MRI contrast agents for the liver [43,44]. The above-mentioned properties of magnetic nanoparticles have enabled their application as MRI contrast agents [45,46], vectors for magnetically targeted drug delivery [5,47–50] and magnetically assisted gene transfection [48,51,52], actuators of cell fate through magnetomechanical actuation of cell surface receptors [27,51,53–57], agents for magnetically triggered drug release [58–61] and in cancer treatment through hyperthermia [4,6,33,34,37,62–64]. These properties make them excellent candidates for the thermal chemosensitization of anticancer drugs, the topic of this review. The following sections will provide a discussion of the mechanisms of heat dissipation of iron oxide-based nanoparticles, current methods and challenges for clinical heat applications, and current work and future perspectives regarding the use of magnetic nanoparticles for the thermal chemopotentiation of therapeutic drugs.
Mechanisms & rates of energy dissipation by magnetic nanoparticles in alternating magnetic fields
Magnetic nanoparticles are attractive for thermal sensitization of cancer cells to chemotherapeutics due to their ability to generate heat at the nanoscale. Understanding the mechanisms of such heat generation may provide the means to optimize and potentially maximize therapeutic outcomes. In the case of iron oxide, single-domain magnetic nanoparticles transform the energy of an applied alternating magnetic field (AMF) into heat by two mechanisms: internal dipole rotation and physical particle rotation (Figure 1). In both cases, there are internal or external factors that prevent the magnetic dipole from following the applied magnetic field, resulting in irreversible energy loss as dissipated heat. The dominant mechanism of energy dissipation depends on a variety of factors, but is expected to correspond to the mechanism with the shortest characteristic relaxation time.
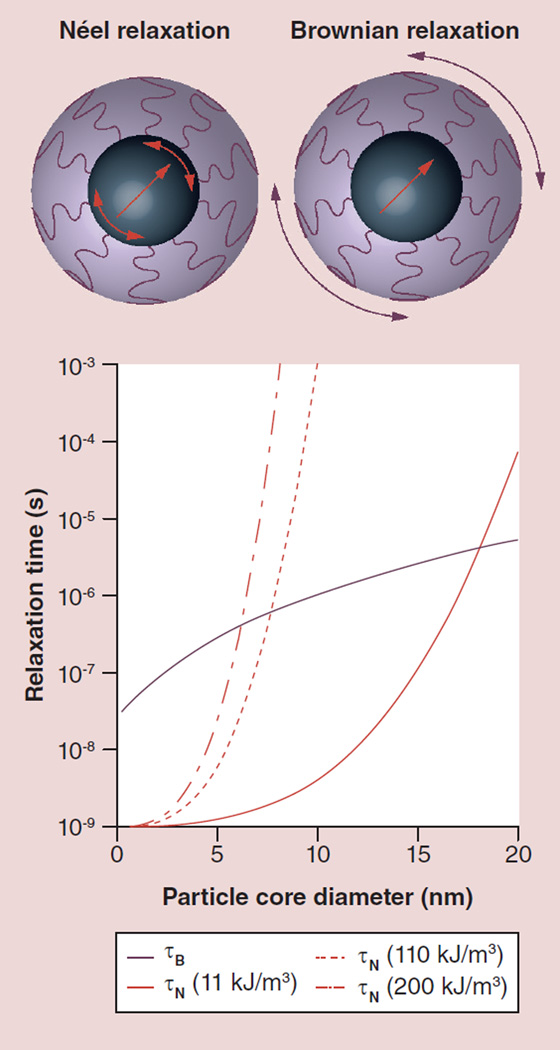
Figure 1. Magnetic nanoparticles respond to time-varying magnetic fields through either internal dipole rotation, so-called Néel relaxation, or through physical particle rotation, so-called Brownian relaxation.
The characteristic relaxation times for each mechanism depend on the particle’s core and hydrodynamic diameter, and the magnetocrystalline anisotropy constant of the magnetic core. Calculations shown here are for room temperature, and for the Brownian mechanism assume that the particles are dispersed in water and possess a 2-nm-thick organic shell. Representative values of the magnetocrystalline anisotropy correspond to bulk magnetite (11 kJ/m3), nanoscale or interacting magnetite (110 kJ/m3) and cobalt ferrite (200 kJ/m3).
τB: Brownian relaxation time; τN: Néel relaxation time.
In the first mechanism of internal dipole rotation, the magnetic dipoles are typically aligned along so-called magnetic easy axes, defined as crystal directions with minimal magnetocrystalline energy. For the dipole to change direction due to an applied AMF, the dipole must surpass an energy barrier, proportional to the so-called magnetocrystalline anisotropy constant and the particle volume. This mechanism is commonly called Néel relaxation and its characteristic relaxation time τN is given by:
| (Equation 1) |
where f0 is a so-called attempt frequency and is commonly assumed to be 109 Hz, K is the magnetocrystalline anisotropy constant, Vc is the volume of the inorganic magnetic core, kB is the Boltzmann’s constant and T is the absolute temperature. The magnetocrystalline anisotropy constant in (Equation 1) depends on the nature of the magnetic material in the nanoparticle and on particle size. For example, for magnetite, a wide range of values, from close to the bulk value of approximately 11 kJ/m3 [65,66] to over an order of magnitude higher [67,68] have been reported.
In the Brownian relaxation mechanism, particles physically rotate to align their dipoles, which are practically fixed along a crystal direction, with the magnetic field. In this case, viscous drag opposes rotation of the particle and leads to dissipation of mechanical energy in the form of heat in the fluid surrounding the nanoparticles. This mechanism is commonly called Brownian relaxation and its characteristic relaxation time τB is given by:
| (Equation 2) |
where η is the viscosity of the fluid surrounding the particles and Vh is the hydrodynamic volume of the particles.
The dominant mechanism for energy dissipation will be the one corresponding to the shorter relaxation time. Due to their distinct dependence on particle diameter, magnetocrystalline anisotropy and medium viscosity, particles below a certain critical size relaxation proceed by the Néel mechanism and above that critical size relaxation proceed by the Brownian mechanism. Figure 1 shows calculated relaxation times for the Néel and Brownian relaxation mechanisms for magnetic nanoparticles as a function of core diameter. Close to this critical diameter the particles will relax by a combination of the two mechanisms and, hence, energy dissipation will occur through a combination of the two mechanisms. Calculations of the Néel relaxation time were made for three distinct values of the magnetocrystalline anisotropy: 11 kJ/m3, a value representative of bulk magnetite [66]; 110 kJ/m3, a value that is an order of magnitude higher and is representative of measurements for nanoscale magnetite and for samples with magnetic interactions [68]; and 200 kJ/m3, a value that is representative of cobalt ferrite [69]. As can be seen in Figure 1, the value of the critical diameter for transition from one dominant mechanism to another depends on the relative values of magnetocrystalline anisotropy and medium viscosity. Of these, one could control magnetocrystalline anisotropy through selection of the magnetic material used in the nanoparticle or by using core–shell geometries. However, care must be taken to select materials with uncompromised biocompatibility if the intended application is biomedical. It is also important to realize that in a collection of particles with a wide size distribution there will be particles both above and below the threshold diameter for switching of the dominant relaxation mechanism; therefore, polydisperse collections of particles are likely to dissipate heat through a mixture of the Néel and Brownian mechanisms.
According to a theoretical calculation by Rosensweig [70], the energy dissipation rate for a given applied field amplitude and frequency can be optimized through judicious selection of particle size, modulation of magnetic relaxation time and selection of the magnetic material that the particles are composed of. This has motivated many recent studies seeking to enhance the energy dissipation rate, of which we highlight a few.
Various authors have considered changing the magnetic material used to make the nanoparticles from iron oxide to other magnetic materials, such as cobalt ferrite [71–73] or core–shell manganese oxide and cobalt ferrite structures [74]. The use of cobalt ferrite yields particles with predominantly Brownian relaxation mechanisms and with relaxation times that are close to the inverse of the typical frequencies used in magnetic fluid hyperthermia (MFH). This leads to enhanced energy dissipation. However, the intrinsic toxicity of cobalt [75] must be taken into account, along with the expectation that nanoparticles that accumulate in tissues will remain there for prolonged periods and may degrade, releasing potentially toxic cobalt ions. Furthermore, because energy dissipation by the Brownian mechanism requires physical particle rotation, under certain conditions, such as entrapment in the extracellular matrix, hindered rotation could lead to significantly lower energy dissipation rates, which is undesirable [76]. Similar arguments regarding toxicity apply to core–shell structures consisting of cobalt ferrite and manganese ferrite that have been shown to have remarkable rates of energy dissipation [77].
More recently, attention has shifted to controlled aggregation of iron oxide nanoparticles to tune particle–particle interactions, thereby increasing the effective magnetocrystalline anisotropy constant. This, in turn, shifts the optimal dissipation frequency to the typical range applied in MFH, enhancing energy dissipation. This is the subject of a recent report where energy dissipation rates as high as 2000 W/g are claimed [78]. Furthermore, one must realize that the theory by Rosensweig [70] is strictly applicable to the case of noninteracting magnetic nanoparticles and for AMFs with low amplitudes and frequencies, such that the magnetization response is linear. These assumptions are hardly applicable under actual experimental conditions and, as such, additional work is needed to obtain a more detailed theoretical description of energy dissipation by single-domain magnetic nanoparticles in AMFs.
Clinical aspects of thermal potentiation of chemotherapeutic agents
Current methods & challenges to deliver heat in the clinic
The use of hyperthermia as a therapeutic modality dates back to the origins of medicine. This is not surprising, as our natural body response to pathogens is fever. In the case of cancer, hyperthermia treatments have been explored for more than 5000 years [79], although their translation to the clinic has only occurred during the past few decades. In the USA and Europe combined, there are more than 350 ongoing clinical trials that incorporate hyperthermia as part of the treatment [201,202]. Furthermore, hyperthermia has been explored not only as a treatment, but also as an adjuvant to enhance antineoplastic treatments such as radiotherapy and chemotherapy.
Methods for applying hyperthermia have evolved. Current techniques include ultrasound, radiofrequency, microwaves, infrared radiation, thermoseeds and hot water. These can be classified according to the extent of the area they can treat. The most common are regional or whole-body heat delivery systems, which have been reviewed in detail elsewhere [80,81]. Some of these may require invasive application to deliver homogeneous heating to the target area. However, to date, the need for heat delivery devices that could reach hyperthermia temperatures within the desired volume still remains. Therefore, the utilization of magnetic nanoparticles as a potential source to generate heat at the nanoscale has tremendous potential.
Magnetic nanoparticles as an alternative to heat delivery
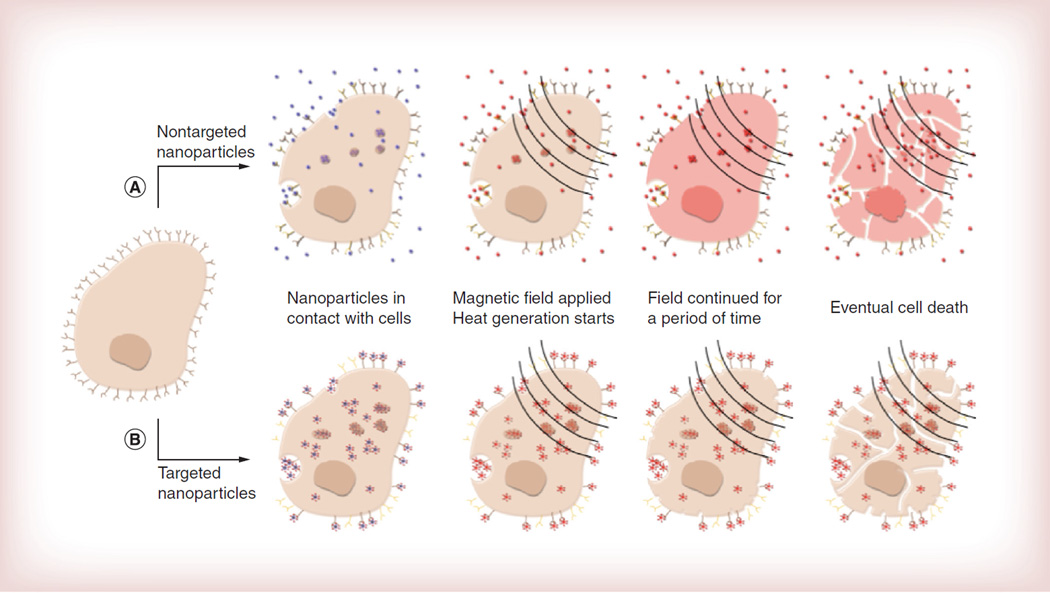
The term MFH is used to describe a form of heat delivery where tissue temperature rises to a therapeutically relevant hyperthermia range (43–47°C) due to the actuation of magnetic nanoparticles under the influence of an AMF. As illustrated in Figure 2A, ideally, magnetic nanoparticles are placed in contact with the desired tissue, either by direct injection or systemically, after which an AMF is applied, heat dissipation by the nanoparticles starts to occur until a high enough thermal dose is applied to cause cell death by various mechanisms.
Figure 2. Progression of magnetic fluid hyperthermia.
(A) Nontargeted magnetic nanoparticles reach the desired cancerous tissue; an alternating magnetic field is applied and nanoparticles dissipate heat; heat dissipation leads to an increase in the temperature of the surroundings reaching hyperthermia levels; and cell death eventually occurs (cell breakage is for illustrative purposes). (B) Targeted nanoparticles are internalized due the presence of a targeting ligand through vesicles, endosomes and lysosomes; an alternating magnetic field is applied only to cells with internalized nanoparticles; and cell death can occur through various mechanisms. A temperature rise may not be observed in the bulk.
MFH is attractive because of the possibility of targeted nanoscale energy delivery to deep tissues using magnetic nanoparticles [82]. It is envisioned that magnetic nanoparticles could either be directly injected into cancer tumors or delivered intravenously and targeted to tumors by a combination of the enhanced permeation and retention effect [83–90] and active targeting through surface ligands [91]. This could result in the localization of nanoparticles in the extracellular matrix surrounding cancer cells, or in cellular uptake and accumulation in intracellular structures such as vesicles, endosomes and lysosomes (Figure 2B).
Application of an AMF would lead to energy dissipation by the nanoparticles [70], potentially raising the tissue temperature to the therapeutically relevant hyperthermia range. Perhaps more exciting is the possibility that, due to intracellular localization of the nanoparticles, MFH could have enhanced anticancer activity over other ‘external’ modes of heat application. In this respect, Gordon hypothesized that internalized magnetic particles could result in a greater local temperature rise within cells or intracellular compartments [92]. However, the possibility of local nanoscale thermal effects due to magnetic nanoparticles in AMFs is perhaps the most controversial technical aspect of MFH. The controversy stems from a fundamental disagreement between expectations based on the macroscopic continuum theory of heat transfer, which indicates that there should be no advantage to delivering heat using nanoparticles, and a growing body of experimental evidence demonstrating chemical and biological outcomes that are influenced or enhanced due to energy delivery by magnetic nanoparticles. Indeed, theoretical analyses by Rabin [93] and Keblinski et al. [94] indicate that the immediate vicinity of an energy-dissipating nanoparticle should not suffer from a preferential increase in temperature. Furthermore, according to heat transfer models, the competition between energy deposition by the nanoparticles and heat removal by conduction in tissue and perfusion of blood through the vasculature gives rise to a lower limit for the size of a tumor that can be brought to the hyperthermia range using a given combination of nanoparticle energy dissipation rate and loading in the tissue [93,95]. This is a serious limitation, as it would preclude application of MFH in the treatment of early-stage tumors or metastatic disease. On the other hand, there is substantial experimental evidence supporting the notion that nanoscale energy delivery by magnetic nanoparticles gives rise to local effects and enhanced biological response. Examples of this are:
-
▪
Evidence of differences in temperature sensed by thermosensitive fluorophores either free in solution or bound to magnetic nanoparticles [27];
-
▪
Observations of a thermally induced transition of a thermoresponsive fluorescent polymer bound to the surface of magnetic nanoparticles immediately upon application of an AMF, despite the fact that the bulk temperature is up to 12°C below the characteristic transition temperature for the polymer [96];
-
▪
Observation of permeabilization of liposomes below their melting temperature when heat is delivered by magnetic nanoparticles localized to the liposome’s lipid bilayer [59];
-
▪
Demonstrations of enhanced reductions in cell viability when subjected to MFH, compared with treatment with external heating under the same thermal dose [97];
-
▪
Observations of enhanced potentiation of anticancer drugs by hyperthermia induced by magnetic nanoparticles, compared with treatment with external heating under the same thermal dose [98];
-
▪
Demonstration that internalized, targeted magnetic nanoparticles can lead to significant reductions in cell viability without a macroscopically perceptible temperature rise [37];
-
▪
Demonstration that targeted nanoparticles accumulating in lysosomes lead to selective disruption of the lysosomal membrane upon application of an AMF without a temperature rise [99].
Taken together, there seems to be overwhelming experimental support for an advantage to deliver heat at the nanoscale using magnetic nanoparticles, even if the exact mechanisms (both physical and biological) for the observed enhanced outcomes remain unknown. Perhaps the macroscopic continuum heat transfer arguments break down at the nanoscale, as has been observed, for example, for the Stokes–Einstein diffusivity of nanoparticles in complex fluids [100]. Or perhaps the above experimental observations require re-interpretation in another context besides differences in temperature.
There are other nanoparticle platforms being investigated for their potential to achieve hyperthermia or thermal ablation to treat cancer and perhaps the most developed is the application of so-called photothermal therapy using gold nanoparticles, nanorods and/or nanoshells. This technology is the subject of several recent reviews [101–106]. Compared with MFH, photothermal therapy has the advantage of being able to deliver thermal energy at much greater rates. However, this requires optical access to the intended area, limiting its potential to treat deep tissues due to absorption and dispersion of light by tissue. Furthermore, as the required light must be focused to achieve energy deposition, photothermal therapy requires knowledge of where the cancer tissue is located and each site must be sequentially treated, making whole-body application difficult. By contrast, because living tissues are relatively transparent to magnetic fields of the frequencies and amplitudes used in MFH, one could potentially treat the whole body using commercially available whole-body AMF applicators [107], or could treat selected regions using properly designed coils. In this sense, one could envision systemic targeted delivery of magnetic nanoparticles to all cancer tissues accessible through the vasculature, followed by simultaneous treatment without the need to individually identify the tumor masses. This could potentially be of great use in treating metastatic disease.
The other major potential advantage of using magnetic nanoparticles for the hyperthermic treatment of cancer has been mentioned multiple times already: the possibility of both passive and active targeting of systemically delivered magnetic nanoparticles to cancerous tumors and cells. The idea is to develop particles whose physicochemical properties enable their extended circulation in the bloodstream and their uptake by cancer tissues and cells through the local leaky vasculature of a growing tumor (i.e., through the so-called enhanced permeation and retention effect) [84]. The further modification of nanoparticles with targeting agents could lead to an enhancement in their uptake and potentially control their intracellular localization. Since magnetic nanoparticles have been under development for a variety of applications since the invention of ferrofluids in the 1960s, there is an extensive knowledge base with respect to their synthesis and surface modification [108–112]. Thus, it was expected that the synthesis and modification of magnetic nanoparticles to achieve selective deposition in targeted tissues such as cancer would be easily achievable. However, this has become one of the greatest obstacles to date.
As noted above, heat transfer arguments indicate that there is a minimum tumor size that can be elevated to the hyperthermia range for a given nanoparticle energy dissipation rate and particle loading. Corollary to this would be the fact that there is a minimum nanoparticle loading required to effectively achieve hyperthermia in a particular tumor using nanoparticles with a given energy dissipation rate. Thus, the potential advantages and challenges of energy delivery by magnetic nanoparticles are coupled with any potential advantages and challenges in their targeted delivery to the intended tissues.
Despite the many demonstrations of the in vitro efficacy of MFH in killing cancer cells, there are relatively few demonstrations in vivo, and most of these are only for the case of local injection of nanoparticles [6,7,113]. Although this route of delivery can certainly be of clinical use, the true potential of MFH can only be realized by targeted systemic delivery of the nanoparticles to all cancer tissues simultaneously. Although there are some demonstrations of targeted systemic delivery of magnetic nanoparticles to cancer tissues, for example in their use as MRI contrast agents, there is limited evidence of successful treatment of tumors in vivo following systemic delivery of targeted magnetic nanoparticles. The challenge here is achieving sufficient nanoparticle loading in the intended tissue to raise the temperature to the hyperthermia range. Perhaps this challenge could be circumvented with improved understanding of the mechanisms by which cancer cells can be destroyed using targeted magnetic nanoparticles without the need for a macroscopic perceptible temperature rise [37]. Furthermore, in addition to the challenge of enhancing magnetic nanoparticle delivery to tissues to be treated by MFH, one must also minimize nonspecific uptake in other important organs, such as the liver, if MFH is to be effectively used as a whole-body application. This is the case illustrated by recent work investigating the negative impact of nonspecific magnetic nanoparticle uptake in murine livers, followed by whole-body application of AMFs [9]. Thus, regardless of intense research, the design of magnetic nanoparticles for systemic targeted delivery to cancer tissues remains a challenge.
The potential advantages and challenges described above are also relevant to the thermal potentiation of chemotherapeutics through hyperthermia induced by magnetic nanoparticles. For this particular application, additional advantages and/or challenges can be envisioned. One potential advantage is that synergy between energy delivery by magnetic nanoparticles and chemotherapeutics could result in a positive therapeutic outcome even if the traditional hyperthermia range of 43–47°C is not achieved. This would somewhat relax the requirements of nanoparticle delivery to intended tissues and/or allow for treatment of smaller tumors, depending on the degree of synergy. However, a potential challenge would be the selective codelivery of the magnetic nanoparticles and chemotherapeutics. Here one could envision multiple strategies, such as: administration of the magnetic nanoparticles and chemotherapeutic separately (either simultaneously or in stages), leading to independent accumulation in the intended tissues (and in nonintended tissues); design of magnetic nanoparticles for the targeted transport of a passively released chemotherapeutic and for local dissipation of energy; and design of magnetic nanoparticles for targeted delivery of a chemotherapeutic whose release is triggered by local energy dissipation. This is still an area that requires further analysis and most likely the best option will differ depending on the chemotherapeutic drug used.
Sensitization of antineoplastics by heat
In order to understand the potential advantages of using MFH as a prospective adjuvant for chemotherapy, it is necessary to understand the challenges involved and the advantages of using heat to promote chemosensitivity. It is well known that the chemosensitivity of tumors is a complex phenomenon. In fact, although new antineoplastic agents have become available in recent decades, these agents alone have not necessarily increased the cure rate of a significant portion of cancers [114]. Advances in the understanding of the underlying cellular mechanisms of therapeutic action have led to the development of drug treatment combinations that pursue optimum therapeutic outcomes. Details of such methodologies have been provided elsewhere [114]. Although improved therapeutic outcomes have been found, further clinical improvement is not expected.
Drug treatment combinations pursue optimized outcomes by trying to obtain pharmacological results that are better than the addition of the outcomes of each individual drug. This idea is the basis of the concept of synergy. Very often this concept has been used interchangeably with drug enhancement. To establish synergy, a detailed and quantitative examination of the effects of each drug’s concentration and treatment sequence [114,115] must be established. Several mathematical methods, including isobolograms and combination indexes have been developed for the examination of synergy in drug combinations [116,117]. To achieve such quantitative assessments, a significant number of experiments must be conducted. In the case of combination treatments of drugs with heat or radiotherapy, the concepts of thermo- and radio-chemosensitization are often used instead of the term synergy. The reason resides in the complexity of the development of a quantitative assessment of synergy given the fact that these two phenomena depend upon many variables including temperature, exposure time, exposure sequence and drug concentration [115,118].
An alternative to quantifying thermochemo-sensitization is defined as the thermal enhancement ratio. This parameter has been defined as the dose required to induce a certain level of cytotoxicity without heat, compared with the dose necessary to induce the same level of cytotoxicity in the presence of heat [115]. Interestingly, this ratio is not necessarily linear in nature, thus illustrating the complexity of the phenomenon [115]. Given the fact that not all chemotherapeutic drugs have been investigated specifically in combination with heat to establish synergy, the word thermochemosensitization will be employed herein to define a pharmacological enhancement caused by the presence of heat, regardless of the source.
To understand the underlying mechanisms of thermochemosensitization it is important to recognize the fundamental influence of thermal effects in an organism. The overall in vivo outcome is a complex relationship between thermal effects from the system to the cellular level. Comprehending the cellular and molecular changes experienced during hyperthermia is of the utmost importance for the optimization of any heat treatment. The essence of heat effects on cell death is based on the inability of the cell to control macromolecular insults, mainly protein unfolding and aggregation, which results in apoptosis or mitotic catastrophe [119]. Specifically, it has been proposed that increased membrane permeability, increased rate of DNA damage, inhibition of DNA repair mechanisms, improved tumor perfusion in vivo, and the possibility that the in vivo hypoxic and acidic environment of the tumor are key factors that may promote selective sensitivity [120–124]. However, these factors must be further analyzed in order to understand how the method of heat application can further improve treatment.
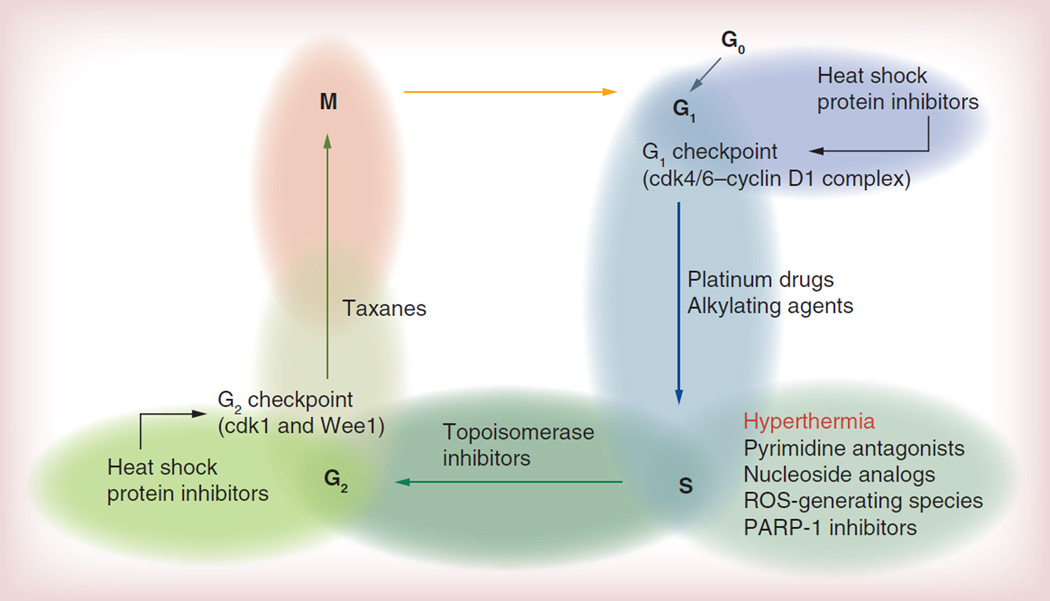
One often overlooked yet important aspect is understanding the effect of the drug on the various stages of the cell cycle. Cells continuously undergo a series of events that eventually lead to division and replication. The most important steps are the G1, S, G2 and M phase. G0 is a resting phase in which the cell is not dividing. In the G1 phase, the cell will increase in size and checkpoint proteins will be synthesized to ensure the integrity of the DNA. If DNA integrity is acceptable, DNA replication occurs during the S phase. After replication, the cell continues to grow and another group of checkpoint proteins will be synthesized to ensure that the division phase can proceed (G2 phase). Finally, cell division occurs (M phase). Figure 3 depicts the cell cycle, and indicates in which steps the most common families of chemotherapeutic agents cause enough damage to induce cell death. Table 1 provides a more detailed summary of various families of chemotherapeutic agents, described according to their effect on the cell cycle, mode of action and potentiation capacity with heat. Table 1 does not include all drugs that have been tested in conjunction with hyperthermia, but rather it is a summary of the chemotherapeutics most commonly investigated.
Figure 3. Cell cycle and potential proteins that require heat shock protein 90 for proper function.
ROS: Reactive oxygen species.
Table 1.
Common antineoplastics tested in combination with heat.
| Classification | Common name | Effect on cell cycle |
Mode of action | Synergy with heat |
Ref. |
|---|---|---|---|---|---|
| Platinum | Cisplatin Oxaliplatin Carboplatin |
G1/S | Formation of DNA adducts | More than additive | [121] |
| Taxanes | Paclitaxel Docetaxel |
G2/M | Microtubule stabilization | Complex (depends on cell type) | [121] |
| Campothecin | Irinotecan Topotecan |
S/G2 | Topoisomerase I inhibitor | [161] | |
| Pyrimidine antagonists | 5-fluorouracil Cytarabine (Ara C) |
S | DNA inhibition | Independent | [81,115,118] |
| Dihydropyrimidine dehydrogenase inhibitor | Gimeracil | S | Inhibits DNA double-strand break repair | [162] | |
| Nucleoside analogs | Gemcitabine | S | DNA inhibition | [132,133,163,144] | |
| Alkylating agents | Cyclophosphamide Mephalan Ifosfamide Mitomycin C |
G1/S | Incorporates an alkyl group to DNA guanine’s bases | More than additive | [81,118] |
| Vinca alcaloids | Vincristin Vinblastine |
M | Mitotic inhibitor that prevents microtubule assembly | Independent | [121] |
| Proteasome inhibitors | Bortezomib | G2/M | Binds to 26S proteasome | More than additive | [126,127,164] |
| ROS-generating species | Tert-butyl hydroperoxide | S | More than additive | [165–167] | |
| Heat shock protein Inhibitors | Geldanamycin Flavonoids Tanespimycin |
Effect on G1 and G2 checkpoint proteins | Decrease in the amount of checkpoint proteins produced | More than additive | [129,134–136] |
| PARP-1 inhibitors | NU1025 PJ-34 |
S | Repair inhibition of single-strand breaks | [168] | |
| Antibiotics | Bleomycin Doxorubicin Actinomycin D |
Breaks DNA DNA intercalation DNA transcription inhibitor | Complex | [81,118] |
ROS: Reactive oxygen species.
Taking into consideration that the main effects of hyperthermia in the cell cycle occur in the S phase (Figure 3), it could be argued that antineoplastics, such as pyrimidine antagonists (i.e., 5-fluoracil), vinca alkaloids (i.e., vinblastine) and taxanes should not demonstrate significant improvements [114,121,125] when treated with hyperthermia because their main mechanism of action occurs in steps of the cell cycle in the S phase or later. For these drugs, ineffective combination treatments are likely to be explained by the concept of cell cycle resistance, as proposed by Shah [114]. This concept proposes that drug resistance, in particular when more than one therapeutic agent is used, can occur due to the impact of one therapeutic agent in the cell cycle rendering the following agent ineffective [114]. When examined closely, it can be observed that the highest potential for thermochemosensitization occurs in those therapeutic agents for which the main mode of action occurs on or before the S phase.
There are some exceptions to the aforementioned rule. Take for example the case of proteasome inhibitors. Bortezomib, a FDA-approved proteasome inhibitor, has demonstrated heat potentiation in vitro and in vivo [126]. Its mode of action occurs in the G2/M phase of the cell cycle [126,127]. Similar to other chemotherapeutic drugs, combination treatments have been explored. Unfortunately, results have demonstrated no further clinical benefit [128]. In the case of a combination treatment with hyperthermia, the fact that cells are chemosensitized by heat indicates that treatment order is essential. It is expected that proteasome inhibition prior to hyperthermia will provide the most potent combination, as significant protein unfolding will overwhelm the compromised proteasome.
Another interesting factor that could be used to explain the lack of thermochemosensitization between certain drugs and hyperthermia is the role of heat shock proteins (HSPs). These proteins, also known as chaperones, are part of the cell’s heat shock response mechanism. They are involved in the response to stresses that could threaten the cell’s survival [129]. The synthesis of HSPs is not only stimulated by heat, but also by any stress-causing agent such as membrane-fluidizing compounds. One could argue that if heat promotes membrane permeabilization, when used in conjunction with chemotherapeutic drugs the concentration will increase, thus promoting cell death. This has not necessarily been the case. For example, Balogh et al. and Dempsey et al. demonstrated the lack of thermochemosensitization of some drugs with fluidizing agents such as MFH [130,131]. They proposed that this could be explained by the upregulation of HSPs. Therefore, depending on cell and drug type, hyperthermia could either promote or inhibit cell death.
Adachi et al. proposed that the activation of HSP70 by hyperthermia inhibited the activation of NF-κB in pancreatic cancer cells, which resulted in an enhanced cytotoxicity of gemcitabine [132]. Again, treatment order was found to be important as prior or post-treatment with hyperthermia enhanced the therapeutic response, but when treatment was performed at the same time an improved response was not observed. Similar results were found when this treatment was tested in a clinical study [133].
In order to elucidate the role of HSPs in the potentiation of heat treatments, several researchers have tested various HSP inhibitors, including quercetin (HSP70 inhibitor) [134], tanespimycin (HSP90 inhibitor) [128], and geldanamycin and its derivatives (HSP90 inhibitors) [129,135,136]. Results have indicated that the combination of HSP inhibitors and hyperthermia potentiates heat treatment. However, it is interesting to note that the previous analysis related to the cell cycle can also be applied to HSP inhibitors. Although their role in the cell cycle is complex and still not well understood, it is believed that the synthesis of several checkpoint proteins relies on the proper function of HSP70 and 90 (Figure 3) [129,137]. Their inhibition would then decrease the amount of checkpoint proteins, leading to potential problems with the successful termination of the cell cycle.
Although the mechanisms of thermochemosensitization are complex and still not well understood, the lessons learned with conventional hyperthermia methods could be applied to MFH. The capability of MFH to deliver heat at specific sites should be exploited and optimized.
Chemopotentiation using heat dissipated by magnetic nanoparticles
Nanotechnology-based systems have been employed to deliver heat to specific target areas for combination therapy or to use the nanoparticle platform to improve drug uptake. This section focuses on the utilization of magnetic nanoparticles for the thermochemosensitization of chemotherapeutic agents. Although many drugs could benefit from combined heat treatments, in the case of MFH, platinum-based drugs have been commonly employed as model drugs for the examination of controlled release and thermochemosensitization. These and other drugs tested in combination with MFH are summarized in Table 2.
Table 2.
Use of magnetic nanoparticles for the thermopotentiation of chemotherapeutic drugs.
| Drug | Iron oxide-based nanoparticle systems |
Purpose | Experimental system | Results | Ref. |
|---|---|---|---|---|---|
| Adriamycin | Nanoparticles conjugated with multidrug resistance protein inhibitors | Combination treatment | In vivo experiments with human chronic myeloid leukemia cell lines | Significant decrease in tumor sizes | [158] |
| Bleomycin | Coprecipitated chitosan-coated nanoparticles | Drug delivery | Release kinetics. Cellular experiments conducted in HeLa cells | Effective release. Increased drug activity found in nanoparticle– drug conjugated systems | [142] |
| Bortezomib | Coprecipitated nanoparticles coated with covalently attached carboxymethyl dextran | Combination treatment | Cellular experiments with resistant and nonresistant cell lines | Combination treatment with MFH more effective in both resistant and nonresistant cell lines when compared with hot water | [160] |
| Carboplatin | Iron nanopowder in chitosan nanoparticles | Combination treatment | In vivo studies for liver carcinoma | Higher survival rates with combination therapy | [156] |
| Cisplatin | Nanoparticles coated with adsorbed starch polymers | Combination treatment | Cellular combination experiments using BP6 rat sarcoma cells | Combination treatment more effective | [148] |
| Magnetic nanoparticles encapsulated in poly(glycolic acid) nanoparticles | Drug delivery by polymer biodegradation and heat | Drug release using microwave pulses | Effective actuated drug release | [145] | |
| Nanoparticles prepared by electrochemical deposition | MDR inhibitors | In vitro studies with SKOV-3/DPP cells | Enhanced accumulation of platinum using nanoparticles | [146] | |
| Coprecipitated nanoparticles coated with adsorbed starch polymers | Drug delivery | Release kineticsc | Cisplatin desorption after magnetic field application | [149] | |
| Porous hollow nanoparticles functionalized with herceptin | Targeted release | Release kinetics and in vitro cellular studies with SK-BR-03 cells | Release kinetics controlled by pH. Significant cytotoxicity found | [150] | |
| Carbon-encapsulated nanoparticles | Combination treatment | Release and cellular studies with DU-145 cells | Combination treatment was effective | [147] | |
| Coprecipitated nanoparticles coated with adsorbed carboxymethyl dextran | Combination treatment | Cellular efficacy studies using Caco-2. Compared with hot water hyperthermia | Treatment sequence is important. Significant differences between heating methods | [151] | |
| Nanoparticles coated with crosslinked starch | Drug delivery | Release kinetics | Release profiles influenced by crosslinking density, pH and temperature | [141] | |
| Coprecipitated gold-coated nanoparticles | Drug delivery/targeted delivery | Cellular studies with resistant and native A2780 cells | Cisplatin-coated nanoparticles demonstrated higher activity | [139] | |
| Coprecipated nanoparticles coated with poly(lactic acid) | Drug delivery | Loading and release kinetics | Effective drug loading. Half of loaded drug was released | [138] | |
| Coprecipitated nanoparticles coated with adsorbed carboxymethyl dextran | Combination treatment/understanding of potentiation mecahnisms | Cellular studies using Caco-2 | No cytoprotective role from copper when used with MFH. Higher platinum uptake and membrane permeability with MFH when compared with hot water | [152] | |
| Concanavalin A | Coprecipitated chitosan-coated nanoparticles | Drug delivery | Release kinetics. Cellular experiments conducted in HeLa cells | Increased drug activity found in nanoparticle–drug conjugated systems | [142] |
| Cyclophosphamide | Citrate-coated nanoparticles | MRI contrast/combination treatment | In vivo experiments in mammary adenocarcinoma | Lifespan of mice significantly improved. MRI enhancement observed | [155] |
| CO3O4−Fe3O4 hybrid nanoparticles | Drug delivery | Release kinetics. Cellular experiments conducted in mouse fibroblast | Controlled release was achieved. Particles were not cytotoxic | [143] | |
| Geldanamycin | Micrometer-sized particles | Combination treatment | In vitro and in vivo studies using B16 melanoma | In vivo, 55% remission in treated group | [157] |
| Gemcitabine | Coprecipitated nanoparticles coated with a polyelectrolyte complex of poly(acrylic acid), chitosan and folic acid | Drug delivery | Release kinetics. Cellular experiments with PRF-5, DLD-1 and MDA-231 cell lines | Nanoparticles delivered the drug to the nuclei of cells | [144] |
| Melphalan | Adsorbed dextran-coated nanoparticles | MRI contrast/combination treatment | In vivo experiments in P388 tumors, Ehlich carcinoma and Lewis carcinoma | Lifespan of mice significantly improved. MRI enhancement observed | [154] |
| Paclitaxel | Nanoparticles encapsulated in liposomes | Combination treatment | Release kinetics. Cellular experiments with HeLa cells | Combination treatment more effective | [159] |
| Selol | Encapsulated magnetic nanoparticles in poly(lactic)-co-glycolic acid nanoparticles | Drug delivery | Cellular studies using B16–F10 cells | Loaded particles produced higher cell cytotoxicity | [140] |
| Quercetin | Combination treatment | In vivo experiments with a melanoma model | Combination treatment more effective | [169] | |
MDR: Multidrug-resistance; MFH: Magnetic fluid hyperthermia.
Recent studies have reported the utilization of iron-based nanoparticle systems as potential drug delivery devices for cisplatin (cDDP). In some cases, the heat produced by the nanoparticle is the trigger for cDDP delivery, while, in others, particles are employed to improve uptake and allow imaging through MRI. For example, Devi-coated coprecipitated iron oxide nanoparticles with poly(lactic acid) [138]. Cisplatin loading and release was investigated without the application of a magnetic field. Similarly, Wagstaff reported a composite nanoparticle system composed of iron oxide nanoparticles coated with gold and covalently attached cDDP [139]. cDDP-containing nanoparticles were found to be more cytotoxic in resistant and native A2780 cells. Other studies have also reported the release of cisplatin from magnetic nanoparticles [140,141] and other drugs, including bleomycin [142], concanavalin A [142], cyclophosphamide [143] and gemcitabine [144].
Naik et al. employed iron oxide nanoparticles encapsulated in poly(glycolic acid) nanoparticles that also included cDDP [145]. Complete release of cDDP was obtained after various 2.4-GHz microwave pulses. These studies did not include any in vivo or cellular experiments to test for effectiveness. Jiang et al. examined the utilization of iron oxide nanoparticles as drug delivery carriers for cDDP and proposed that these nanoparticles could serve as MDR inhibitors [146]. In vitro studies with SKOV-3/DPP cells indicated that, although statistically significant, the increase in platinum uptake in the presence of the nanoparticles was only 23%. In this particular case, heat or magnetic field was not applied.
Several iron-based magnetic nanoparticles have been employed for the thermochemosensitization of antineoplastic agents. Carbonencapsulated iron oxide magnetic nanoparticles were developed and investigated by Taylor et al. [147]. Cellular studies were conducted in DU-145 cells using cisplatin and MFH, and the authors concluded that the combined treatment was more effective. Babincová et al. examined iron oxide nanoparticles functionalized with starch domains for the ionic binding and controlled release of cDDP [148]. In vitro tests in BP6 cells revealed that enhanced effects were obtained when combined treatments were examined. Similarly, Kettering et al. provided a systematic study of particle stability and release under various environmental conditions for the system employed by Babincová, indicating that release profiles were a function of environmental properties, such as pH and protein presence [149].
A targeted system was developed by Cheng et al., who designed porous, hollow, magnetic nanoparticles functionalized with herceptin for the targeted release of cDDP [150]. In vitro, release kinetics were pH dependent. Tests performed with SK-BR-03 cells indicated a significant reduction in cell viability when targeted nanoparticles were employed.
More recently, Lee et al. [151] and Alvarez-Berrios et al. [152] investigated the utilization of iron oxide magnetic nanoparticles for the thermochemosensitization of cisplatin in vitro using the Caco-2 cell model. Lee et al. reported the effects of treatment sequence and heating technique [151]. The effects of treatment sequence were evident for both heat application methods, which were hot water and MFH. The highest cytotoxicity was observed when cDDP and hyperthermia were applied at the same time with an additional exposure period with the drug. Similar behavior was observed for both hot water and MFH. However, it is interesting to note that when both heat modalities were compared, MFH was more effective for all treatment sequences when compared with heat applied by a hot water bath under the same conditions. Similar observations were reported by Rodriguez-Luccioni et al. for the case of MFH without drugs [153]. The aforementioned results may indicate that membrane permeabilization could possess a significant role and is dependent on the type of heat treatment. To address this issue, Alvarez-Berrios et al. investigated the effect of membrane permeabilization on the potentiation of cDDP by MFH [152]. In this particular case, copper was employed to minimize the active transport of cDDP via the hCTR1 receptor. Results indicated that copper did not demonstrate any cytoprotective role when MFH was applied, whereas it did protect those cells that were treated with a hot water bath. Furthermore, platinum uptake experiments and fluorescence anisotropy measurements indicated that higher drug uptake and membrane permeabilization was obtained when MFH was applied. These results indicate that MFH may not only improve drug cytotoxicity by increasing drug concentration through membrane permeabilization, but other mechanisms may be at hand. Such mechanisms are complex and have not yet been elucidated.
Brusentsov et al. employed dextran ferrite- and citrate-coated magnetic nanoparticles and tested them for MRI contrast in combination with MFH with melphalan and cyclophosphamide in vivo using P388 tumors, Ehlich carcinoma, Lewis lung carcinoma and mammary adenocarcinoma [154,155]. Their results indicated that the lifespan of mice was considerably improved with significant tumor remission. They also claimed that MRI enhancement provided the means to identify tumor metastasis in all cases. In this particular case, details regarding the method of magnetic field application were not clear.
Recently, Li et al. encapsulated carboplatin and iron nanopowder in chitosan nanoparticles [156]. They performed in vivo studies for liver carcinoma, where particles were directly injected into the hepatic artery and a magnetic field was applied. Results indicated that significant survival rates were obtained for those groups treated with combination therapy in comparison to MFH alone.
Thermochemosensitization using magnetic nanoparticles has also been investigated with other drugs. These include HSP, PARP and proteasome inhibitors. Ito et al. investigated the combined heat treatment with geldanamycin, an HSP 90 inhibitor, with MFH in vitro and in vivo using a B16 melanoma model [157]. The particles employed were iron-based particles of 100 µm in diameter. In vivo results demonstrated complete remission in five out of nine mice treated with the combined treatment. More recently, Ren et al. investigated the thermochemosensitization of adriamycin using iron oxide nanoparticles functionalized with multidrug resistance protein inhibitors in vivo [158]. The results indicated that the combination treatment using multidrug resistance protein inhibitor-conjugated nanoparticles demonstrated significant decreases in tumor size when compared with those that did not. Paclitaxel-loaded magnetoliposomes were tested in vitro in HeLa cancer cells [159], showing that combination treatment was more effective.
Alvarez et al. investigated the in vitro thermal enhancement of bortezomib by MFH in resistant and nonresistant cells [160]. Bortezomib has been approved by the FDA for the treatment of multiple myeloma, but its use has been limited due to high clinical toxicity, and combination treatments have not provided additional benefits [126,128]. Results demonstrated decreased cell survival in those cells treated with bortezomib and MFH. Combination treatment with magnetic nanoparticles resulted in decreased cell survival when compared with a similar treatment performed under the same thermal doses using hot water hyperthermia. Interestingly, cells that demonstrated resistance to bortezomib were, in fact, more sensitive to treatment than nonresistant cells. This reveals that MFH can also sensitize otherwise drug-resistant cells.
The utilization of magnetic nanoparticles for the generation of targeted heat has tremendous potential, not only to improve current treatments, but also to serve as an alternative to improve the therapeutic window of drugs that otherwise have shown clinical toxicity or no further clinical improvements. The mechanisms by which such improvements occur are complex and merit further investigation.
Conclusion & future perspective
The use of magnetic nanoparticles to generate heat at the nanoscale with the purpose of cancer treatment either alone or as an adjuvant for chemotherapy has an exciting future. The mechanisms of thermochemosensitization are complex and still not well understood. The lessons learned with conventional hyperthermia methods could be applied to MFH. This is certainly an area that must be addressed in order to optimize potential therapeutic outcomes. Furthermore, although the use of magnetic nanoparticles is attractive, there are still challenges that must be addressed and understood in order to successfully translate such technology to the clinic. These include: improving particle loading in the tumor tissue by selecting appropriate targets but minimizing nonspecific accumulation in relevant organs; improving particle heat generation; and optimizing in vivo drug and heat administration strategies. With these in mind, one can foresee that, within the next couple of years, effective targeting strategies will be further developed, including the use of aptamers and other ligands as well as in-depth understanding of local nanoscale thermal effects. With such advances, one can anticipate that within the next decade significant progress towards the development of systemically delivered externally actuated and magnetically controlled nanodelivery devices will be made. These nanoparticle platforms will not only recognize and accumulate in the desired diseased tissue, but will also improve the therapeutic index of the selected drugs. One can only dream of such platforms specifically tailored to the needs of each individual patient.
Executive summary.
Magnetic nanoparticles & magnetic fluid hyperthermia
-
▪
Magnetic nanoparticles are small inorganic crystals coated or embedded in an organic shell that possess superparamagnetic, ferromagnetic or ferromagnetic behavior.
-
▪
They respond to applied magnetic fields by translation, physical particle rotation or internal dipole rotation, converting magnetic field energy into either mechanical forces/torque or thermal energy.
-
▪
In general, magnetic nanoparticles are biocompatible.
-
▪
Magnetic fluid hyperthermia (MFH) is defined as a form of treatment where therapeutically relevant temperatures (43–47°C) are obtained through the actuation of magnetic nanoparticles.
-
▪
Magnetic nanoparticles are small enough that they could be either directly injected into cancers or intravenously delivered.
-
▪
MFH is an attractive form of therapy because energy at the nanoscale can be targeted to tissues.
Mechanisms & rates of energy dissipation by magnetic nanoparticles in alternating magnetic fields
-
▪
Iron oxide nanoparticles transform applied magnetic energy by internal dipole rotation (Néel) and physical rotation (Brownian).
-
▪
The energy dissipation rate for a given field and amplitude can be manipulated by the selection of particle size, magnetic relaxation time and the composition of nanoparticles.
Clinical aspects of thermal potentiation of chemotherapeutic drugs
-
▪
Hyperthermia modalities are various, including ultrasound, radiofrequency, microwaves, infrared radiation, thermoseeds and hot water.
-
▪
Not all therapeutic drugs demonstrate improvements in therapeutic outcome in the presence of heat.
-
▪
Thermal effects in vivo are a complex combination of effects from the cellular to systems level.
-
▪
Hyperthermia affects the cell in the S phase of the cell cycle.
Chemopotentiation using heat dissipated by magnetic nanoparticles
-
▪
Magnetic nanoparticles have been investigated for the local release of chemotherapeutic drugs such as cisplatin.
-
▪
In vitro MFH cellular studies with drugs such as cisplatin have demonstrated that combination treatments using magnetic nanoparticles are more effective than using each individually.
-
▪
In vivo MFH experiments in combination with drugs have indicated that patient survival and tumor remission can be achieved.
-
▪
Emerging experimental evidence supports the notion that nanoscale thermal effects occur in the vicinity of magnetic nanoparticles in alternating magnetic fields, and that these effects can lead to disruption of cellular components and cell death.
-
▪
Localized thermal effects could enhance thermal chemopotentiation of therapeutic drugs.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪of considerable interest
- 1.Cornell RM, Schwertmann U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. 2nd Edition. Germany: Wiley-VCH Verlag GMBH & Co, Weinheim; 2003. [Google Scholar]
- 2.Spaldin NA. Magnetic Materials: Fundamentals and Applications. 2nd Edition. NY, USA: Cambridge University Press; 2010. [Google Scholar]
- 3.Russel WB, Saville DA, Schowalter WR. Colloidal Dispersions. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- 4.Jordan A, Scholz R, Wust P, Fähling H, Felix R. Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999;201(1):413–419. [Google Scholar]
- 5.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Dennis CL, Jackson AJ, Borchers JA, et al. Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology. 2009;20(39):395103. doi: 10.1088/0957-4484/20/39/395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giustini AJ, Ivkov R, Hoopes PJ. Magnetic nanoparticle biodistribution following intratumoral administration. Nanotechnology. 2011;22(34):345101. doi: 10.1088/0957-4484/22/34/345101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khot VM, Salunkhe AB, Thorat ND, Ningthoujam RS, Pawar SH. Induction heating studies of dextran coated MgFe2O4 nanoparticles for magnetic hyperthermia. Dalton Trans. 2012;42(4):1249. doi: 10.1039/c2dt31114c. [DOI] [PubMed] [Google Scholar]
- 9. Kut C, Zhang Y, Hedayati M, et al. Preliminary study of injury from heating systemically delivered, nontargeted dextran–superparamagnetic iron oxide nanoparticles in mice. Nanomedicine. 2012;7(11):1697–1711. doi: 10.2217/nnm.12.65. ▪ Illustrates the importance of targeted delivery and minimizing nonspecific accumulation as it could lead to side effects and morbidity during whole-body application of an alternating magnetic field.
- 10.Easo SL, Mohanan PV. Dextran stabilized iron oxide nanoparticles: synthesis, characterization and in vitro studies. Carbohydr. Polym. 2013;92(1):726–732. doi: 10.1016/j.carbpol.2012.09.098. [DOI] [PubMed] [Google Scholar]
- 11.Herrera AP, Barrera C, Rinaldi C. Synthesis and functionalization of magnetite nanoparticles with aminopropylsilane and carboxymethyldextran. J. Mater. Chem. 2008;18(31):3650–3654. [Google Scholar]
- 12.Creixell M, Herrera AP, Latorre-Esteves M, Ayala V, Torres-Lugo M, Rinaldi C. The effect of grafting method on the colloidal stability and in vitro cytotoxicity of carboxymethyl dextran coated magnetic nanoparticles. J. Mater. Chem. 2010;20(39):8539–8547. [Google Scholar]
- 13.López-Cruz A, Barrera C, Calero-DdelC VL, Rinaldi C. Water dispersible iron oxide nanoparticles coated with covalently linked chitosan. J. Mater. Chem. 2009;19(37):6870–6876. [Google Scholar]
- 14.Wang J, Zhao G, Li Y, Liu X, Hou P. Reversible immobilization of glucoamylase onto magnetic chitosan nanocarriers. Appl. Microbiol. Biotechnol. 2012;97(2):681–692. doi: 10.1007/s00253-012-3979-2. [DOI] [PubMed] [Google Scholar]
- 15.Hoque SM, Srivastava C, Srivastava N, Venkateshan N, Chattopadhyay K. Synthesis and characterization of Fe- and Co-based ferrite nanoparticles and study of the T1 and T2 relaxivity of chitosan-coated particles. J. Mater. Sci. 2012;48(2):812–818. [Google Scholar]
- 16.Unsoy G, Yalcin S, Khodadust R, Gunduz G, Gunduz U. Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. J. Nanopart. Res. 2012;14(11):964. [Google Scholar]
- 17.Sonvico F, Mornet S, Vasseur S, et al. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: synthesis, physicochemical characterization, and in vitro experiments. BioconJ. Chem. 2005;16(5):1181–1188. doi: 10.1021/bc050050z. [DOI] [PubMed] [Google Scholar]
- 18.Sun C, Sze R, Zhang M. Folic acid–PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J. Biomed. Mater. Res. Part A. 2006;78A(3):550–557. doi: 10.1002/jbm.a.30781. [DOI] [PubMed] [Google Scholar]
- 19.Barrera C, Herrera A, Zayas Y, Rinaldi C. Surface modification of magnetite nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2009;321(10):1397–1399. [Google Scholar]
- 20.Barrera C, Herrera AP, Rinaldi C. Colloidal dispersions of monodisperse magnetite nanoparticles modified with poly(ethylene glycol) J. Colloid Interface Sci. 2009;329(1):107–113. doi: 10.1016/j.jcis.2008.09.071. [DOI] [PubMed] [Google Scholar]
- 21.Barrera C, Herrera AP, Bezares N, et al. Effect of poly(ethylene oxide)-silane graft molecular weight on the colloidal properties of iron oxide nanoparticles for biomedical applications. J. Colloid Interface Sci. 2012;377(1):40–50. doi: 10.1016/j.jcis.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 22.Bee A, Massart R, Neveu S. Synthesis of very fine maghemite particles. J. Magn. Magn. Mater. 1995;149(1):6–9. [Google Scholar]
- 23.Halbreich A, Roger J, Pons JN, et al. Biomedical applications of maghemite ferrofluid. Biochimie. 1998;80(5):379–390. doi: 10.1016/s0300-9084(00)80006-1. [DOI] [PubMed] [Google Scholar]
- 24.RuizMoreno RG, Martinez AI, Castro-Rodriguez R, Bartolo P. Synthesis and characterization of citrate coated magnetite nanoparticles. J. Supercond. Nov. Magn. 2012;26(3):709–712. [Google Scholar]
- 25.Ruizmoreno RG, Martínez AI, Falcony C, Castro-Rodriguez R, Bartolo-Pérez P, Castro-Román M. One pot synthesis of water compatible and monodisperse magnetite nanoparticles. Mater. Lett. 2013;92(C):181–183. [Google Scholar]
- 26.Fauconnier N, Pons JN, Roger J, Bee A. Thiolation of maghemite nanoparticles by dimercaptosuccinic acid. J. Colloid Interface Sci. 1997;194(2):427–433. doi: 10.1006/jcis.1997.5125. [DOI] [PubMed] [Google Scholar]
- 27. Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 2010;5(8):602–606. doi: 10.1038/nnano.2010.125. ▪▪ Provides direct experimental evidence of nanoscale thermal effects in the vicinity of magnetic nanoparticles in an alternating magnetic field.
- 28.Xiong F, Zhu ZY, Xiong C, et al. Preparation, characterization of 2-deoxy-d-glucose functionalized dimercaptosuccinic acid-coated maghemite nanoparticles for targeting tumor cells. Pharm. Res. 2011;29(4):1087–1097. doi: 10.1007/s11095-011-0653-9. [DOI] [PubMed] [Google Scholar]
- 29.Konda SD, Aref M, Wang S, Brechbiel M, Wiener EC. Specific targeting of folate-dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magn. Reson. Mater. Phys. Biol. Med. 2001;12(2–3):104–113. doi: 10.1007/BF02668091. [DOI] [PubMed] [Google Scholar]
- 30.Yoo MK, Park IK, Lim HT, et al. Folate–PEG–superparamagnetic iron oxide nanoparticles for lung cancer imaging. Acta Biomater. 2012;8(8):3005–3013. doi: 10.1016/j.actbio.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Mahajan S, Koul V, Choudhary V, Shishodia G, Bharti AC. Preparation and in vitro evaluation of folate-receptor-targeted SPION–polymer micelle hybrids for MRI contrast enhancement in cancer imaging. Nanotechnology. 2012;24(1) doi: 10.1088/0957-4484/24/1/015603. 015603. [DOI] [PubMed] [Google Scholar]
- 32.Ahrens ET, Feili-Hariri M, Xu H, Genove G, Morel PA. Receptor-mediated endocytosis of iron-oxide particles provides efficient labeling of dendritic cells for in vivo MR imaging. Magn. Reson. Med. 2003;49(6):1006–1013. doi: 10.1002/mrm.10465. [DOI] [PubMed] [Google Scholar]
- 33.Grüttner C, Müller K, Teller J, Westphal F, Foreman A, Ivkov R. Synthesis and antibody conjugation of magnetic nanoparticles with improved specific power absorption rates for alternating magnetic field cancer therapy. J. Magn. Magn. Mater. 2007;311(1):181–186. [Google Scholar]
- 34.Le B, Shinkai M, Kitade T, et al. Preparation of tumor-specific magnetoliposomes and their application for hyperthermia. J. Chem. Eng. Jap. 2001;34(1):66–72. [Google Scholar]
- 35.Yang L, Mao H, Wang YA, et al. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small. 2008;5(2):235–243. doi: 10.1002/smll.200800714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Creixell M, Herrera AP, Ayala V, et al. Preparation of epidermal growth factor (EGF) conjugated iron oxide nanoparticles and their internalization into colon cancer cells. J. Magn. Magn. Mater. 2010;322(15):2244–2250. [Google Scholar]
- 37. Creixell M, Bohórquez AC, Torres-Lugo M, Rinaldi C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano. 2011;5(9):7124–7129. doi: 10.1021/nn201822b. ▪▪ Demonstrates that significant reductions in cell viability are possible without the need for a macroscopic temperature rise when magnetic nanoparticles are targeted to the EGFR and an alternating magnetic field is applied.
- 38.Wang AZ, Bagalkot V, Vasilliou CC, et al. Superparamagnetic iron oxide nanoparticle–aptamer bioconjugates for combined prostate cancer imaging and therapy. ChemMedChem. 2008;3(9):1311–1315. doi: 10.1002/cmdc.200800091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu MK, Kim D, Lee IH, So JS, Jeong YY, Jon S. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small. 2011;7(15):2241–2249. doi: 10.1002/smll.201100472. [DOI] [PubMed] [Google Scholar]
- 40.Bamrungsap S, Shukoor MI, Chen T, Sefah K, Tan W. Detection of lysozyme magnetic relaxation switches based on aptamer-functionalized superparamagnetic nanoparticles. Anal. Chem. 2011;83(20):7795–7799. doi: 10.1021/ac201442a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bamrungsap S, Chen T, Shukoor MI, et al. Pattern recognition of cancer cells using aptamer-conjugated magnetic nanoparticles. ACS Nano. 2012;6(5):3974–3981. doi: 10.1021/nn3002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auerbach M. Ferumoxytol as a new, safer, easier-to-ad minister intravenous iron: yes or no? Am. J. Kidney Dis. 2008;52(5):826–829. doi: 10.1053/j.ajkd.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Marincek B. Diagnostic improvement in MRI of gynecological neoplasms. J. Belge Radiol. 1996;79(1):13–17. [PubMed] [Google Scholar]
- 44.Reimer P, Balzer T. Ferucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applications. Eur. Radiol. 2003;13(6):1266–1276. doi: 10.1007/s00330-002-1721-7. [DOI] [PubMed] [Google Scholar]
- 45.Sun C, Lee J, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Del. Rev. 2008;60(11):1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Na HB, Song IC, Hyeon T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009;21(21):2133–2148. [Google Scholar]
- 47.Arruebo M, Fernández-Pacheco R, Ibarra MR, Santamaria J. Magnetic nanoparticles for drug delivery. Nano Today. 2007;2(3):22–32. [Google Scholar]
- 48.Mcbain SC, Yiu HHP, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomedicine. 2008;3(2):169. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tietze R, Lyer S, Dürr S, Alexiou C. Nanoparticles for cancer therapy using magnetic forces. Nanomedicine (Lond.) 2012;7(3):447–457. doi: 10.2217/nnm.12.10. [DOI] [PubMed] [Google Scholar]
- 50.Wang N, Guan Y, Yang L, et al. Magnetic nanoparticles (MNPs) covalently coated by PEO-PPO-PEO block copolymer for drug delivery. J. Colloid Interface Sci. 2013;395(C):50–57. doi: 10.1016/j.jcis.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 51.Pankhurst QA, Thanh NTK, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009;42(22):224001. [Google Scholar]
- 52.Schwerdt JI, Goya GF, Calatayud P, Hereñú CB, Reggiani PC, Goya RG. Magnetic field-assisted gene delivery: achievements and therapeutic potential. Curr. Gene Ther. 2012;12(2):116–126. doi: 10.2174/156652312800099616. [DOI] [PubMed] [Google Scholar]
- 53.Mannix RJ, Kumar S, Cassiola F, et al. Nanomagnetic actuation of receptor-mediated signal transduction. Nat. Nanotechnol. 2007;3(1):36–40. doi: 10.1038/nnano.2007.418. [DOI] [PubMed] [Google Scholar]
- 54.Dobson J. Remote control of cellular behaviour with magnetic nanoparticles. Nature Nanotechnol. 2008;3:139–143. doi: 10.1038/nnano.2008.39. [DOI] [PubMed] [Google Scholar]
- 55.Lee JH, Kim ES, Cho MH, et al. Artificial control of cell signaling and growth by magnetic nanoparticles. Angew Chem. Int. Ed. 2010;49(33):5698–5702. doi: 10.1002/anie.201001149. [DOI] [PubMed] [Google Scholar]
- 56.Sniadecki NJ. Minireview: a tiny touch: activation of cell signaling pathways with magnetic nanoparticles. Endocrinology. 2010;151(2):451–457. doi: 10.1210/en.2009-0932. [DOI] [PubMed] [Google Scholar]
- 57.Cho MH. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat. Mater. 2012;11(12):1038–1043. doi: 10.1038/nmat3430. [DOI] [PubMed] [Google Scholar]
- 58.Derfus AM, von Maltzahn G, Harris TJ, et al. Remotely triggered release from magnetic nanoparticles. Adv. Mater. 2007;19(22):3932–3936. [Google Scholar]
- 59.Amstad E, Kohlbrecher J, Müller E, Schweizer T, Textor M, Reimhult E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett. 2011;11(4):1664–1670. doi: 10.1021/nl2001499. [DOI] [PubMed] [Google Scholar]
- 60.Hu SH, Chen YY, Liu TC, Tung TH, Liu DM, Chen SY. Remotely nano-rupturable yolk/shell capsules for magnetically-triggered drug release. Chem. Commun. 2011;47(6):1776. doi: 10.1039/c0cc03998e. [DOI] [PubMed] [Google Scholar]
- 61.Amstad E, Reimhult E. Nanoparticle actuated hollow drug delivery vehicles. Nanomedicine. 2012;7(1):145–164. doi: 10.2217/nnm.11.167. [DOI] [PubMed] [Google Scholar]
- 62.Dennis CL, Jackson AJ, Borchers JA, et al. The influence of collective behavior on the magnetic and heating properties of iron oxide nanoparticles. J. Appl. Phys. 2008;103(7) 07A319. [Google Scholar]
- 63.Latorre M, Rinaldi C. Applications of magnetic nanoparticles in medicine: magnetic fluid hyperthermia. PR Health Sci. J. 2009;28(3):227–238. [PubMed] [Google Scholar]
- 64.Laurent S, Dutz S, Häfeli UO, Mahmoudi M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011;166(1–2):8–23. doi: 10.1016/j.cis.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Bickford LR. Ferromagnetic resonance absorption in magnetite single crystals. Phys. Review. 1950;78(4):449–457. [Google Scholar]
- 66.Bickford LR, Brownlow JM, Penoyer RF. Magnetocrystalline anisotropy in cobalt-substituted magnetite single crystals. Proc. IEE-Part B Radio Electron. Eng. 1957;104(5 Suppl):238–244. [Google Scholar]
- 67.Goya GF, Berquó TS, Fonseca FC, Morales MP. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003;94(5):3520. [Google Scholar]
- 68.Del Castillo VLCD, Rinaldi C. Effect of sample concentration on the determination of the anisotropy constant of magnetic nanoparticles. IEEE Magn. Trans. 2010;46(3):852–859. [Google Scholar]
- 69.Tung LD, Kolesnichenko V, Caruntu D, Chou NH, O’Connor CJ, Spinu L. Magnetic properties of ultrafine cobalt ferrite particles. J. Appl. Phys. 2003;93(10):7486–7488. [Google Scholar]
- 70. Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002;252:370–374. ▪ Lays the foundations for theoretical calculations of the energy dissipation rate of magnetic nanoparticles and for its optimization by modifying nanoparticle properties.
- 71.Lee SW, Bae S, Takemura Y, et al. Self-heating characteristics of cobalt ferrite nanoparticles for hyperthermia application. J. Magn. Magn. Mater. 2007;310(2):2868–2870. [Google Scholar]
- 72.Veverka M, Veverka P, Kaman O, et al. Magnetic heating by cobalt ferrite nanoparticles. Nanotechnology. 2007;18(34):345704. [Google Scholar]
- 73.Kim DH, Nikles DE, Johnson DT, Brazel CS. Heat generation of aqueously dispersed CoFe2O4 nanoparticles as heating agents for magnetically activated drug delivery and hyperthermia. J. Magn. Magn. Mater. 2008;320(19):2390–2396. [Google Scholar]
- 74.Lee JH, Jang JT, Choi JS, et al. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011;6(7):418–422. doi: 10.1038/nnano.2011.95. [DOI] [PubMed] [Google Scholar]
- 75.Simonsen LO, Harbak H, Bennekou P. Cobalt metabolism and toxicology – a brief update. Sci. Total Environ. 2012;432(C):210–215. doi: 10.1016/j.scitotenv.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 76.Fortin JP, Gazeau F, Wilhelm C. Intracellular heating of living cells through Néel relaxation of magnetic nanoparticles. Eur. Biophys. J. 2007;37(2):223–228. doi: 10.1007/s00249-007-0197-4. [DOI] [PubMed] [Google Scholar]
- 77.Liu G, Hong RY, Guo L, Li YG, Li HZ. Preparation, characterization and MRI application of carboxymethyl dextran coated magnetic nanoparticles. Appl. Surf. Sci. 2011;257(15):6711–6717. [Google Scholar]
- 78. Lartigue L, Hugounenq P, Alloyeau D, et al. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents. ACS Nano. 2012;6(12):10935–10949. doi: 10.1021/nn304477s. ▪ Achieved unprecedented energy dissipation rates in iron oxide nanoparticles through controlled cluster formation.
- 79.Nielsen OS, Horsman M, Overgaard J. A future for hyperthermia in cancer treatment? Eur. J. Cancer. 2001;37(13):1587–1589. doi: 10.1016/s0959-8049(01)00193-9. [DOI] [PubMed] [Google Scholar]
- 80.Franckena M, van der Zee J. Use of combined radiation and hyperthermia for gynecological cancer. Curr. Opin. Obstet. Gynecol. 2010;22(1):9–14. doi: 10.1097/GCO.0b013e328333d1e2. [DOI] [PubMed] [Google Scholar]
- 81.Pennacchioli E, Fiore M, Gronchi A. Hyperthermia as an adjunctive treatment for soft-tissue sarcoma. Expert Rev. Anticancer Ther. 2009;9(2):199–210. doi: 10.1586/14737140.9.2.199. [DOI] [PubMed] [Google Scholar]
- 82.Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann. Surg. 1957;146(4):596–606. doi: 10.1097/00000658-195710000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 84.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001;41(41):189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 85.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Del. Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 86.Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J. Control. Release. 2012;164(2):108–114. doi: 10.1016/j.jconrel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J. Control. Release. 2012;161(2):175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 88.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J. Control. Release. 2012;164(2):138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 89.Taurin S, Nehoff H, Greish K. Anticancer nanomedicine and tumor vascular permeability; where is the missing link? J. Control. Release. 2012;164(3):265–275. doi: 10.1016/j.jconrel.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 90.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Del. Rev. 2013;65(1):71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Alexis F, Rhee JW, Richie JP, Radovic-Moreno AF, Langer R, Farokhzad OC. New frontiers in nanotechnology for cancer treatment. Urol. Oncol. 2008;26(1):74–85. doi: 10.1016/j.urolonc.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 92.Gordon RT, Hines JR, Gordon D. Intracellular hyperthermia – biophysical approach to cancer-treatment via intracellular temperature and biophysical alterations. Med. Hypotheses. 1979;5(1):83–102. doi: 10.1016/0306-9877(79)90063-x. [DOI] [PubMed] [Google Scholar]
- 93. Rabin Y. Is intracellular hyperthermia superior to extracellular hyperthermia in the thermal sense? Int. J. Hyperthermia. 2002;18(3):194–202. doi: 10.1080/02656730110116713. ▪▪ Theoretical calculations of the temperature profiles in a tumor cell and surrounding a magnetic nanoparticle, indicating that there are no advantages to delivering thermal energy using nanoparticles.
- 94.Keblinski P, Cahill DG, Bodapati A, Sullivan CR, Taton TA. Limits of localized heating by electromagnetically excited nanoparticles. J. Appl. Phys. 2006;100(5) 054305. [Google Scholar]
- 95.Andrä W, d’Ambly CG, Hergt R, Hilger I, Kaiser WA. Temperature distribution as function of time around a small spherical heat source of local magnetic hyperthermia. J. Magn. Magn. Mater. 1999;194(1):197–203. [Google Scholar]
- 96.Polo-Corrales L, Rinaldi C. Monitoring iron oxide nanoparticle surface temperature in an alternating magnetic field using thermoresponsive fluorescent polymers. J. Appl. Phys. 2012;111(7) 07B334. [Google Scholar]
- 97.Rodriguez HL, Latorre-Esteves M, Mendez J, et al. Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. Int. J. Nanomedicine. 2011;6:373–380. doi: 10.2147/IJN.S14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee JS, Rodriguez-Luccioni HL, Méndez J, et al. Hyperthermia induced by magnetic nanoparticles improves the effectiveness of the anticancer drug cis-diamminedichloroplatinum. J. Nanosci. Nanotechnol. 2011;11(5):4153–4157. doi: 10.1166/jnn.2011.3821. [DOI] [PubMed] [Google Scholar]
- 99.Domenech M, Marrero-Berrios I, Torres-Lugo M, Rinaldi C. Lysosomal membrane permeabilization by targeted magnetic nanoparticles in alternating magnetic fields. ACS Nano. 2013;7(6):5091–5101. doi: 10.1021/nn4007048. [DOI] [PubMed] [Google Scholar]
- 100.Tuteja A, Mackay ME, Narayanan S, Asokan S, Wong MS. Breakdown of the continuum stokes – Einstein relation for nanoparticle diffusion. Nano Lett. 2007;7(5):1276–1281. doi: 10.1021/nl070192x. [DOI] [PubMed] [Google Scholar]
- 101.Liao H, Nehl CL, Hafner JH. Biomedical applications of plasmon resonant metal nanoparticles. Nanomedicine. 2006;1(2):201–208. doi: 10.2217/17435889.1.2.201. [DOI] [PubMed] [Google Scholar]
- 102.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2007;23(3):217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 103.Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc. Chem. Res. 2008;41(12):1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 104.Strong LE, West JL. Thermally responsive polymer-nanoparticle composites for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011;3(3):307–317. doi: 10.1002/wnan.138. [DOI] [PubMed] [Google Scholar]
- 105.Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Del. Rev. 2012;64(2):190–199. doi: 10.1016/j.addr.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 106.Mieszawska AJ, Mulder WJM, Fayad ZA, Cormode DP. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013;10(3):831–847. doi: 10.1021/mp3005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jordan A, Scholz R, Maier-Hauff K, et al. Presentation of a new magnetic field therapy system for the treatment of human solid tumors with magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2001;225(1):118–126. [Google Scholar]
- 108.Hao R, Xing R, Xu Z, Hou Y, Gao S, Sun S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010;22(25):2729–2742. doi: 10.1002/adma.201000260. [DOI] [PubMed] [Google Scholar]
- 109.Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Del. Rev. 2010;62(3):284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ho D, Sun X, Sun S. Monodisperse magnetic nanoparticles for theranostic applications. Acc. Chem. Res. 2011;44(10):875–882. doi: 10.1021/ar200090c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xie J, Liu G, Eden HS, Ai H, Chen X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc. Chem. Res. 2011;44(10):883–892. doi: 10.1021/ar200044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yiu HH. Engineering the multifunctional surface on magnetic nanoparticles for targeted biomedical applications: a chemical approach. Nanomedicine (Lond.) 2011;6(8):1429–1446. doi: 10.2217/nnm.11.132. [DOI] [PubMed] [Google Scholar]
- 113.Johannsen M, Gneveckow U, Taymoorian K, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective Phase I trial. Int. J. Hyperthermia. 2007;23(3):315–323. doi: 10.1080/02656730601175479. [DOI] [PubMed] [Google Scholar]
- 114.Shah MA, Schwartz GK. Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin. Cancer Res. 2001;7(8):2168–2181. [PubMed] [Google Scholar]
- 115.Petin VG, Kim JK, Zhurakovskaya GP, Dergacheva IP. Some general regularities of synergistic interaction of hyperthermia with various physical and inactivating agents. Int. J. Hyperthermia. 2002;18(1):40–49. doi: 10.1080/02656730110087059. [DOI] [PubMed] [Google Scholar]
- 116.Greco WR, Bravo G, Parsons JC. The search for synergy – a critical-review from a response-surface perspective. Pharmacol. Rev. 1995;47(2):331–385. [PubMed] [Google Scholar]
- 117.Berenbaum MC. What is synergy. Pharmacol. Rev. 1989;41(2):93–141. [PubMed] [Google Scholar]
- 118.Kapinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field. Int. J. Hyperthermia. 2006;22(3):191–196. doi: 10.1080/02656730500532028. [DOI] [PubMed] [Google Scholar]
- 119.Roti Roti JL. Cellular responses to hyperthermia (40–46 degrees C): cell killing and molecular events. Int. J. Hyperthermia. 2008;24(1):3–15. doi: 10.1080/02656730701769841. [DOI] [PubMed] [Google Scholar]
- 120.Hauck TS, Jennings TL, Yatsenko T, Kumaradas JC, Chan WCW. Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv. Mater. 2008;20(20):3832–3838. [Google Scholar]
- 121.Issels RD. Hyperthermia adds to chemotherapy. Eur. J. Cancer. 2008;44(17):2546–2554. doi: 10.1016/j.ejca.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 122.Ohtsubo T, Saito H, Tanaka N, et al. Enhancement of cisplatin sensitivity and platinum uptake by 40 degrees C hyperthermia in resistant cells. Cancer Lett. 1997;119(1):47–52. doi: 10.1016/s0304-3835(97)00250-4. [DOI] [PubMed] [Google Scholar]
- 123.Takemoto M, Kuroda M, Urano M, et al. The effect of various chemotherapeutic agents given with mild hyperthermia on different types of tumours. Int. J. Hyperthermia. 2003;19(2):193–203. doi: 10.1080/0265673021000035235. [DOI] [PubMed] [Google Scholar]
- 124.van Bree C, Franken NA, Snel FA, Haveman J, Bakker PJ. Wild-type p53-function is not required for hyperthermia-enhanced cytotoxicity of cisplatin. Int. J. Hyperthermia. 2001;17(4):337–346. doi: 10.1080/02656730110053137. [DOI] [PubMed] [Google Scholar]
- 125.Peng CL, Tsai HM, Yang SJ, et al. Development of thermosensitive poly(N-isopropylacrylamide-co-((2-dimethylamino) ethyl methacrylate))-based nanoparticles for controlled drug release. Nanotechnology. 2011;22(26):265608. doi: 10.1088/0957-4484/22/26/265608. [DOI] [PubMed] [Google Scholar]
- 126.Neznanov N, Komarov AP, Neznanova L, Stanhope-Baker P, Gudkov AV. Proteotoxic stress targeted therapy (PSTT): induction of protein misfolding enhances the antitumor effect of the proteasome inhibitor bortezomib. Oncotarget. 2011;2(3):209–221. doi: 10.18632/oncotarget.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Piperdi B, Ling YH, Liebes L, Muggia F, Perez-Soler R. Bortezomib: understanding the mechanism of action. Mol. Cancer Ther. 2011;10(11):2029–2030. doi: 10.1158/1535-7163.MCT-11-0745. [DOI] [PubMed] [Google Scholar]
- 128.Kapoor P, Ramakrishnan V, Rajkumar SV. Bortezomib combination therapy in multiple myeloma. Semin. Hematol. 2012;49(3):228–242. doi: 10.1053/j.seminhematol.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johnson VA, Singh EK, Nazarova LA, Alexander LD, McAlpine SR. Macrocyclic inhibitors of Hsp90. Curr. Top. Med. Chem. 2010;10(14):1380–1402. doi: 10.2174/156802610792232088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Balogh G, Maulucci G, Gombos I, et al. Heat stress causes spatially-distinct membrane re-modelling in K562 leukemia cells. PLoS One. 2011;6(6):e21182. doi: 10.1371/journal.pone.0021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dempsey NC, Ireland HE, Smith CM, Hoyle CF, Williams JHH. Heat shock protein translocation induced by membrane fluidization increases tumor-cell sensitivity to chemotherapeutic drugs. Cancer Lett. 2010;296(2):257–267. doi: 10.1016/j.canlet.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 132.Adachi S, Kokura S, Okayama T, et al. Effect of hyperthermia combined with gemcitabine on apoptotic cell death in cultured human pancreatic cancer cell lines. Int. J. Hyperthermia. 2009;25(3):210–219. doi: 10.1080/02656730802657036. [DOI] [PubMed] [Google Scholar]
- 133.Ishikawa T, Kokura S, Sakamoto N, et al. Phase II trial of combined regional hyperthermia and gemcitabine for locally advanced or metastatic pancreatic cancer. Int. J. Hyperthermia. 2012;28(7):597–604. doi: 10.3109/02656736.2012.695428. [DOI] [PubMed] [Google Scholar]
- 134.Rusak G, Gutzeit HO, Ludwig-Muller J. Effects of structurally related flavonoids on hsp gene expression in human promyeloid leukaemia cells. Food Technol. Biotechnol. 2002;40(4):267–273. [Google Scholar]
- 135.Grant S. Enhancing proteotoxic stress as an anticancer strategy. Oncotarget. 2011;2(4):284–286. doi: 10.18632/oncotarget.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yavelsky V, Vais O, Piura B, Wolfson M, Rabinovich A, Fraifeld V. The role of Hsp90 in cell response to hyperthermia. J. Therm. Biol. 2004;29(7–8):509–514. [Google Scholar]
- 137.Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif. 2000;33(6):341–365. doi: 10.1046/j.1365-2184.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Devi SV, Prakash T. Kinetics of cisplatin release by in vitro using poly(d,l-lactide) coated Fe3O4 nanocarriers. IEEE Trans. NanoBiosci. 2013;12(1):60–63. doi: 10.1109/TNB.2012.2230024. [DOI] [PubMed] [Google Scholar]
- 139.Wagstaff AJ, Brown SD, Holden MR, et al. Cisplatin drug delivery using gold-coated iron oxide nanoparticles for enhanced tumour targeting with external magnetic fields. Inorg. Chim. Acta. 2012;393:328–333. [Google Scholar]
- 140.Falqueiro AM, Primo FL, Morais PC, Mosiniewicz-Szablewska E, Suchocki P, Tedesco AC. Selol-loaded magnetic nanocapsules: a new approach for hyperthermia cancer therapy. J. Appl. Phys. 2011;109(7) p07B306. [Google Scholar]
- 141.Likhitkar S, Bajpai AK. Magnetically controlled release of cisplatin from superparamagnetic starch nanoparticles. Carbohydr. Polym. 2012;87(1):300–308. doi: 10.1016/j.carbpol.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 142.Kavaz D, Odabas S, Guven E, Demirbilek M, Denkbas EB. Bleomycin loaded magnetic chitosan nanoparticles as multifunctional nanocarriers. J. Bioact. Compatible Polym. 2010;25(3):305–318. [Google Scholar]
- 143.Kumari S, Singh RP. Glycolic acid-functionalized chitosan-CO3O4-Fe3O4 hybrid magnetic nanoparticles-based nanohybrids scaffolds for drug delivery and tissue engineering. J. Mater. Sci. 2013;48:1524–1532. [Google Scholar]
- 144.Viota JL, Carazo A, Munoz-Gamez JA, et al. Functionalized magnetic nanoparticles as vehicles for the delivery of the antitumor drug gemcitabine to tumor cells. Physicochemical in vitro evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2013;33(3):1183–1192. doi: 10.1016/j.msec.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 145.Naik S, Carpenter EE. Poly(d,l-lactide-co-glycolide) microcomposite containing magnetic iron core nanoparticles as a drug carrier. J. Appl. Phys. 2008;103(7) 07A313. [Google Scholar]
- 146.Jiang Z, Chen BA, Xia GH, et al. The reversal effect of magnetic Fe3O4 nanoparticles loaded with cisplatin on SKOV3/DDP ovarian carcinoma cells. Int. J. Nanomedicine. 2009;4(1):107–114. doi: 10.2147/ijn.s5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taylor A, Krupskaya Y, Kramer K, et al. Cisplatin-loaded carbon-encapsulated iron nanoparticles and their in vitro effects in magnetic fluid hyperthermia. Carbon. 2010;48(8):2327–2334. [Google Scholar]
- 148. Babincová M, Altanerova V, Altaner C, Bergemann C, Babinec P. In vitro analysis of cisplatin functionalized magnetic nanoparticles in combined cancer chemotherapy and electromagnetic hyperthermia. IEEE Trans. Nanobiosci. 2008;7(1):15–19. doi: 10.1109/TNB.2008.2000145. ▪ One of the first demonstrations of combination treatments with magnetic fluid hyperthermia.
- 149.Kettering M, Zorn H, Bremer-Streck S, et al. Characterization of iron oxide nanoparticles adsorbed with cisplatin for biomedical applications. Phys. Med. Biol. 2009;54(17):5109–5121. doi: 10.1088/0031-9155/54/17/003. [DOI] [PubMed] [Google Scholar]
- 150.Cheng K, Peng S, Xu CJ, Sun S. Porous hollow Fe3O4 nanoparticles for targeted delivery and controlled release of cisplatin. J. Am. Chem. Soc. 2009;131(30):10637–10644. doi: 10.1021/ja903300f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee JS, Rodríguez-Luccioni HL, Méndez J, et al. Hyperthermia induced by magnetic nanoparticles improves the effectiveness of the anticancer drug cis-diamminedichloroplatinum. J. Nanosci. Nanotechnol. 2011;11(5):4153–4157. doi: 10.1166/jnn.2011.3821. [DOI] [PubMed] [Google Scholar]
- 152.Alvarez-Berríos MP, Castillo A, Méndez J, Soto O, Rinaldi C, Torres-Lugo M. Hyperthermic potentiation of cisplatin by magnetic nanoparticle heaters is correlated with an increase in cell membrane fluidity. Int. J. Nanomedicine. 2013;8:1003–1013. doi: 10.2147/IJN.S38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rodríguez-Luccioni HL, Latorre-Esteves M, Méndez-Vega J, et al. Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. Int. J. Nanomedicine. 2011;6:373–380. doi: 10.2147/IJN.S14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Brusentsov NA, Brusentsova TN, Filinova EY, et al. Magnetohydrodynamic thermochemotherapy and MRI of mouse tumors. J. Magn. Magn. Mater. 2007;311(1):176–180. ▪▪ Demonstrates the in vivo effectiveness of using combination treatments of chemotherapeutic drugs with magnetic fluid hyperthermia.
- 155.Brusentsov NA, Polyanskii VA, Pirogov YA, et al. Antitumor effects of the combination of magnetohydrodynamic thermochemotherapy and magnetic resonance tomography. Pharm. Chem. J. 2010;44(6):291–295. [Google Scholar]
- 156.Li FR, Yan WH, Guo YH, Qi H, Zhou HX. Preparation of carboplatin-Fe@C-loaded chitosan nanoparticles and study on hyperthermia combined with pharmacotherapy for liver cancer. Int. J. Hyperthermia. 2009;25(5):383–391. doi: 10.1080/02656730902834949. [DOI] [PubMed] [Google Scholar]
- 157.Ito A, Saito H, Mitobe K, et al. Inhibition of heat shock protein 90 sensitizes melanoma cells to thermosensitive ferromagnetic particle-mediated hyperthermia with low Curie temperature. Cancer Sci. 2009;100(3):558–564. doi: 10.1111/j.1349-7006.2008.01072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ren YY, Zhang HJ, Chen BA, et al. Multifunctional magnetic Fe3O4 nanoparticles combined with chemotherapy and hyperthermia to overcome multidrug resistance. Int. J. Nanomedicine. 2012;7:2261–2269. doi: 10.2147/IJN.S29357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kulshrestha P, Gogoi M, Bahadur D, Banerjee R. In vitro application of paclitaxel loaded magnetoliposomes for combined chemotherapy and hyperthermia. Colloids Surf. B Biointerfaces. 2012;96:1–7. doi: 10.1016/j.colsurfb.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 160.Alvarez-Berrios MP, Castillo A, Rinaldi C, Torres-Lugo M. Magnetic fluid hyperthermia enhances bortezomib cytotoxicity in sensitive and resistant cancer cell lines. Int. J. Nanomedicine. 2013 doi: 10.2147/IJN.S51435. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann. Surg. Oncol. 2003;10(4):463–468. doi: 10.1245/aso.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 162.Takagi M, Sakata K, Someya M, et al. The combination of hyperthermia or chemotherapy with gimeracil for effective radiosensitization. Strahlenther. Onkol. 2012;188(3):255–261. doi: 10.1007/s00066-011-0043-6. [DOI] [PubMed] [Google Scholar]
- 163.Vertrees RA, Das GC, Popov VL, et al. Synergistic interaction of hyperthermia and gemcitabine in lung cancer. Cancer Biol. Ther. 2005;4(10):1144–1153. doi: 10.4161/cbt.4.10.2074. [DOI] [PubMed] [Google Scholar]
- 164.Chen F, Rezavi R, Wang CC, Harrison LE. Proteasome inhibition potentiates the cytotoxic effects of hyperthermia in HT-29 colon cancer cells through inhibition of heat shock protein 27. Oncology. 2007;73(1–2):98–103. doi: 10.1159/000120997. [DOI] [PubMed] [Google Scholar]
- 165.Chen F, Wang CC, Kim E, Harrison LE. Hyperthermia in combination with oxidative stress induces autophagic cell death in HT-29 colon cancer cells. Cell Biol. Int. 2008;32(7):715–723. doi: 10.1016/j.cellbi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 166.Razavi R, Harrison LE. Thermal sensitization using induced oxidative stress decreases tumor growth in an in vivo model of hyperthermic intraperitoneal perfusion. Ann. Surg. Oncol. 2010;17(1):304–311. doi: 10.1245/s10434-009-0674-3. [DOI] [PubMed] [Google Scholar]
- 167.Wang CC, Chen F, Kim E, Harrison LE. Thermal sensitization through ROS modulation: a strategy to improve the efficacy of hyperthermic intraperitoneal chemotherapy. Surgery. 2007;142(3):384–392. doi: 10.1016/j.surg.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 168.Eppink B, Krawczyk PM, Stap J, Kanaar R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int. J. Hyperthermia. 2012;28(6):509–517. doi: 10.3109/02656736.2012.695427. [DOI] [PubMed] [Google Scholar]
- 169.Miyagawa T, Saito H, Minamiya Y, et al. Inhibition of Hsp90 and 70 sensitizes melanoma cells to hyperthermia using ferromagnetic particles with a low Curie temperature. Int. J. Clin. Oncol. 2013 doi: 10.1007/s10147-013-0606-x. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
Websites
- 201. ClinicalTrial.gov. www.clinicaltrials.gov.
- 202.EU Clinical Trials Registry. www.clinicaltrialsregister.eu. [Google Scholar]