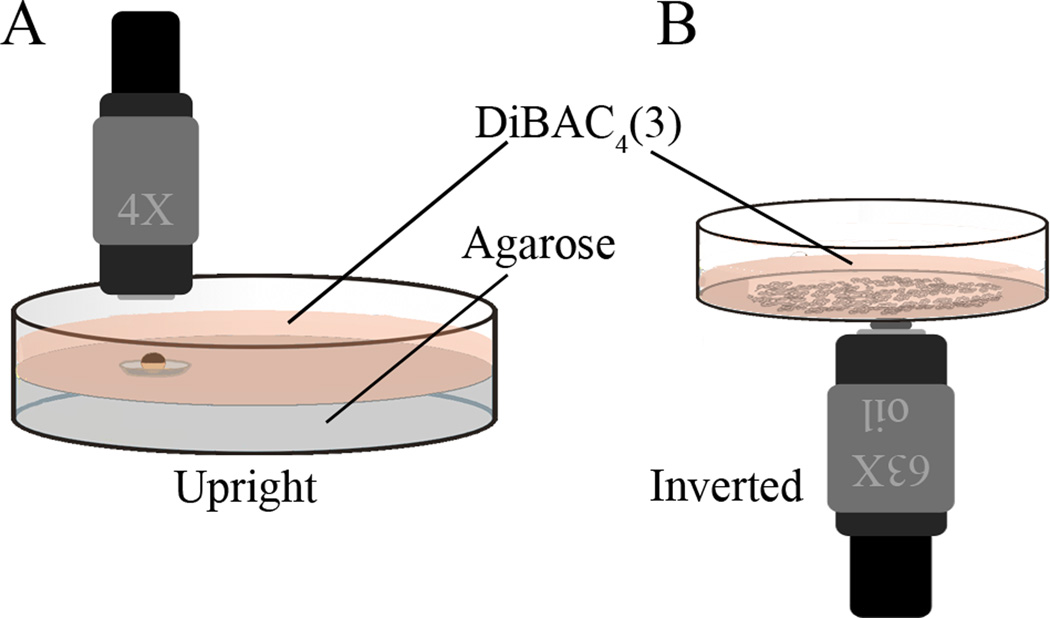
Figure 1.
Dishes for imaging large specimens and cells in culture. (A) A Petri dish with a depression is useful for holding larger, or moving specimens. A convenient way to make these is to pour 2% agarose into a Petri dish; then, before the agarose starts to solidify, float the cap of a microcentrifuge tube, outside up, at the surface. The volume enclosed by the ridge underneath will hold a bubble of air. When the gel is solid, remove the cap and there will be a circular well the diameter of that internal ridge. The caps of 0.5 mL PCR tubes make a depression exactly big enough (5 mm in diameter) to hold seven Xenopus embryos. If shorter working distance lenses will be used, it is useful to fill the dish as high as possible with agarose so that the sample will be near the coverslip but still exposed to the DiBAC solution. (B) The bottom of a FluoroDish is made of coverslip glass, and thus is an excellent surface on which to grow cultured cells for this kind of imaging. If using an inverted microscope, the dish can simply be filled with DiBAC solution; for use on an upright scope, a round coverslip placed over the well will hold enough DiBAC solution for short term imaging with the dish turned upside down, although it should be remembered that it is a much smaller volume and thus bleaching may prove a problem.