Abstract
Objective:
To examine the potential long-term impact of restless legs syndrome (RLS) and other common sleep complaints on subsequent physical function (PF), we conducted a longitudinal analysis of 12,556 men in the Health Professionals Follow-up Study.
Methods:
We used a set of questions recommended by the International RLS Study Group to assess RLS in 2002. We asked questions regarding other sleep complaints—insomnia, sleep fragmentation, and excessive daytime sleepiness—in 2004. We used the Physical Function (PF-10) survey of the Short Form–36 Health Survey to characterize PF in 1996 and 2008. We examined the 2008 PF-10 scores across categories of baseline RLS (2002), adjusted for age, 1996 PF-10 score, and other potential confounders.
Results:
The participants with RLS at baseline had significantly lower PF-10 score 6 years later than those without RLS (mean difference = −2.32, p = 0.01), after adjusting for potential confounders. The magnitude of difference in PF-10 score for RLS symptoms ≥15 times/month vs no RLS was more than that of a 5-year increase of age or moderate amount of smoking. Having daily daytime sleepiness and sleep duration ≥9 hours/day were associated with lower mean PF value than not having these symptoms (p < 0.05 for both).
Conclusions:
RLS and other sleep complaints are associated with lower PF. Our findings need to be replicated by more longitudinal studies including women and populations of other social and cultural backgrounds. It is important to understand whether RLS is an independent risk factor or a marker for other unknown risk factors for disability.
Restless legs syndrome (RLS), also known as Ekbom syndrome, is characterized by a strong, nearly irresistible, urge to move the legs, resulting in marked sleep disturbances. RLS can present with a wide range of severity. Severe RLS is known to be associated with sleep fragmentation, depression, anxiety, obesity, obstructive sleep apnea (OSA), cardiovascular diseases, diabetes, erectile dysfunction, and end-stage renal disease (ESRD).1–3 Individuals with RLS may also have difficulties in job performance and participating in social activities.2,4
The association of RLS and health-related quality of life (HRQOL) has been shown previously.5–9 However, there has been a lack of longitudinal data showing the long-term association of RLS with the individual's physical function (PF) in activities of daily living (ADLs). Our objective was to characterize the longitudinal association of RLS on PF and juxtapose its impact with those of other common diseases, such as hypertension, myocardial infarction (MI), anxiety, and depression. This is a large population-based long-term observational study on individuals' PF, which reflects an individual's ability to live independently and is a validated marker for disability.9–12 The magnitude of RLS-associated disability, i.e., the disease burden on quality of life (QOL) and health care–related cost, in an older-aged population is an important clinical and public health outcome, particularly given the context of an aging population.
METHODS
Population.
The Health Professionals Follow-up Study (HPFS) was established in 1986, when 51,529 male US health professionals aged 40 to 75 years completed a mailed questionnaire about their medical history and lifestyle. Follow-up questionnaires had been mailed to participants every 2 years to update information on potential risk factors and to ascertain newly diagnosed diseases.
Standard protocol approvals, registrations, and patient consents.
The institutional review board at Brigham and Women's Hospital and Harvard School of Public Health reviewed and approved this study, and receipt of each questionnaire implied participant's consent.
Assessment of RLS and other sleep complains.
In 2002, 31,729 men who were still alive and actively participating in the study completed RLS questions, which were developed based on the International RLS Study Group criteria.13,14 We asked the following question: “Do you have unpleasant leg sensations (like crawling, paresthesia, or pain) combined with motor restlessness and an urge to move?” The possible responses were as follows: no; less than once/month; 2 to 4 times/month; 5 to 14 times/month; and 15+ times/month. We asked those who answered that they had any RLS symptoms the following 2 questions: (1) “Do these symptoms occur only at rest and does moving improve them?” and (2) “Are these symptoms worse in the evening/night compared with the morning?” We defined a participant who had symptoms ≥5 times per month and answered yes to the subsequent questions to have RLS; others were classified as without RLS.15
In 2011, we mailed a supplementary RLS questionnaire to the HPFS participants who met the above criteria based on the 2002 questionnaire. The questionnaire included several questions regarding potential mimics of RLS (e.g., positional discomfort and muscle cramps based on the Cambridge–Hopkins RLS questionnaire16), and physician-diagnosed iron-deficiency anemia. In sensitivity analyses, we excluded men who reported to have RLS symptoms always due to positional discomfort or leg cramps, and those who had ever been diagnosed with iron-deficiency anemia.
In 2000, we collected information on snoring frequency with a questionnaire. We defined frequent snoring as snoring every night or most nights. In 2004, we added 4 questions to the questionnaire to characterize other sleep complaints: “How often do you have difficulty falling asleep?” “How often do you have trouble with waking up during the night?” “How often are you troubled by waking up too early and not being able to fall asleep again?” and “How often do you get so sleepy during the day or the evening that you have to take a nap?” The possible responses were as follows: (a) most of the time; (b) sometimes; (c) rarely or never. Answers “most of the time,” “sometimes,” and “rarely or never” were categorized as 2, 1, and 0, respectively, and summed to create a “sleep score.” We considered the scores equal to or above 4 (i.e., the half point of the maximum value) as having a sleep complaint other than RLS or snoring (referred to as “other sleep complaint” in the text).
Assessment of PF.
Among the 28,451 men who completed 2002 RLS questionnaires and were still alive, 21,662 men (76.1%) completed 10 self-administered questions in the Physical Function (PF-10) survey of the Short Form-36 Health Survey (SF-36) in 2008. The PF-10 questionnaire asks whether patients are restricted in the following activities:
Vigorous activities (e.g., running, lifting heavy objects)
Moderate activities (e.g., moving a table, bowling)
Lifting or carrying groceries
Climbing several flights of stairs
Climbing one flight of stairs
Bending, kneeling, or stooping
Walking more than 1 km
Walking several 100 m
Walking 100 m
Bathing or dressing self
Scores on the PF-10 items were summed together and linearly transformed to range between 0 and 100 using the standard algorithm, with higher scores indicating higher function.17
Participants with and without PF-10 information had similar RLS prevalence (4.8% vs 5.5%; p = 0.54). The same questions were also asked in 1996. The SF-36 is a widely used and validated instrument for measuring health status and is independent of any specific disease.9,10,17,18 PF-10, a subset of SF-36, can be administered independently with proven validity, and it has been shown to measure physical activity in daily living with important clinical relevance.17 PF-10 score has been used to assess self-reported QOL among patients with RLS.19,20
To reduce a possible misclassification of RLS, we further excluded men with diabetes and arthritis (n = 9106) up to 2008 in the current analysis, leaving 12,556 participants. Participant inclusion was detailed in figure e-1 on the Neurology® Web site at Neurology.org.
Assessment of covariates.
We collected information on potential confounders, such as age, race, smoking status, weight, height, physical activity, medication use, Crown-Crisp phobic anxiety index, and history of major chronic diseases, including stroke, hypertension, MI, diabetes, arthritis, and Parkinson disease (PD), via biennial questionnaires throughout the follow-up period.
Statistical analyses.
We categorized participants into 3 groups: no RLS, RLS with symptoms 5 to 14 times/month, and RLS with symptoms 15+ times/month. We used multivariate regression models to estimate PF-10 score in 2008 and 95% confidence intervals across categories of RLS status. As a secondary analysis, we also regressed RLS status on the change in PF-10 scores from 1996 to 2008, after adjusting for potential covariates.
Analyses were adjusted for age, race, smoking status, alcohol drinking, body mass index (BMI), baseline physical activity, use of antidepressants (as a surrogate of presence of depression), the Crown-Crisp phobic anxiety index, presence of stroke, hypertension, ESRD, PD, MI, erectile dysfunction, emphysema or chronic bronchitis, chronic obstructive pulmonary disease, pneumonia, asthma, pernicious anemia, ulcerative colitis or Crohn disease, other sleep complaints, and snoring status. In a secondary analysis, we further adjusted for the updated status of the major chronic diseases up to 2008, and the use of antihypertensive, antihistamines, nonsteroidal anti-inflammatory drugs (NSAIDs), and acetaminophen. Because iron deficiency has been found to be associated with RLS,21 we adjusted for dietary iron intake and use of iron-specific supplements in the analysis. To minimize potential residual confounding due to presence of major chronic conditions, we conducted another sensitivity analysis by excluding men with MI, stroke, obesity, PD, cancer, and renal failure. To examine whether other sleep complaints modified the association between RLS and PF score, we tested the interaction between RLS and other sleep complaints in relation to average PF score by adding a multiplicative factor in the model. Furthermore, we tested interactions between RLS and age (<65 vs ≥65 years, approximate mean of the participants), BMI (<25 vs ≥25 kg/m2), smoking (never vs ever), and physical activity (low vs high, based on the median value) in relation to the PF score. Statistical analyses were completed with SAS version 9.1 (SAS Institute, Inc. Cary, NC). All p values were 2-tailed.
RESULTS
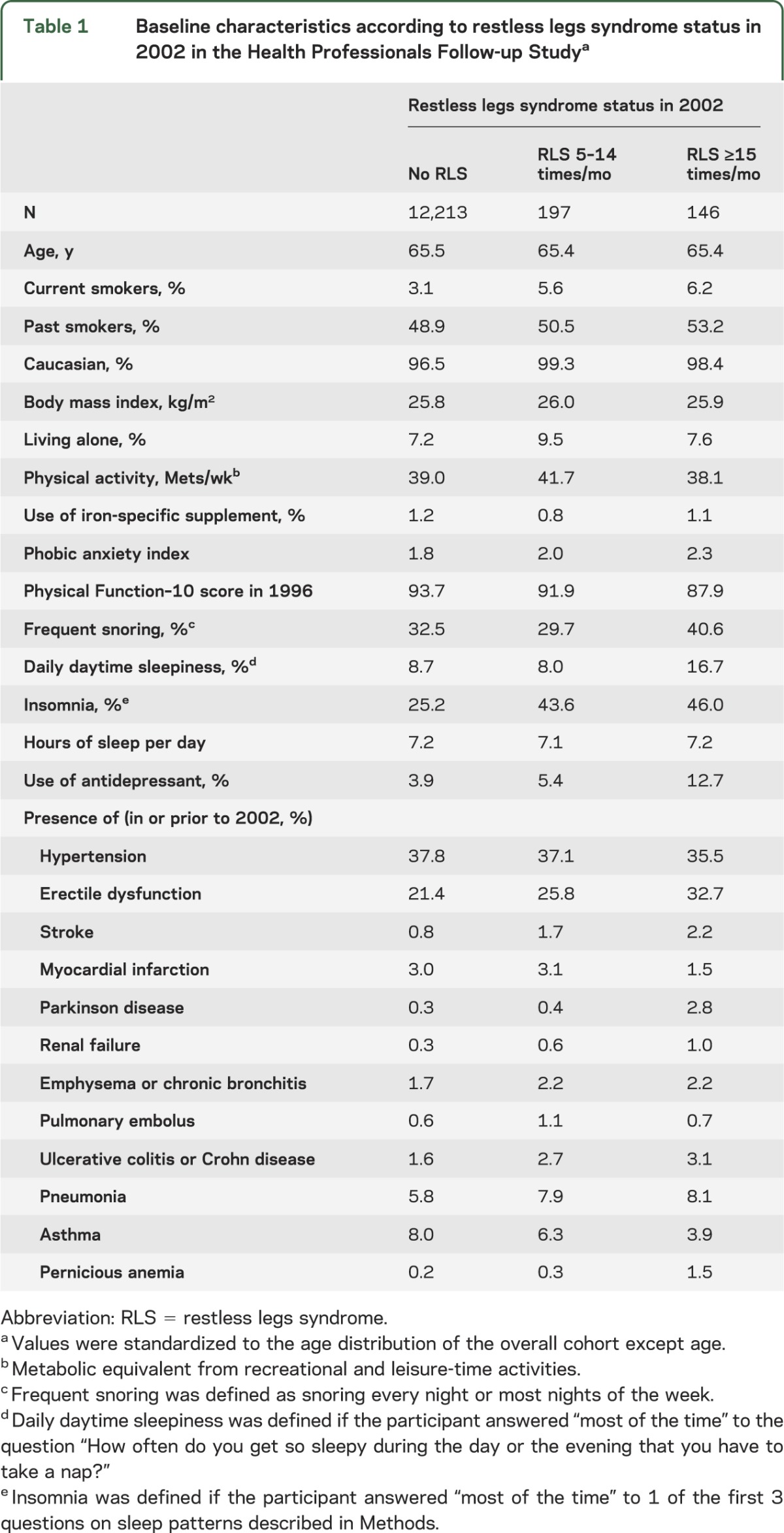
Participants with RLS were more likely to be smokers, live alone at home, have insomnia, use antidepressants, have had a stroke, have PD, have renal failure, or have daily daytime sleepiness, relative to those without RLS (table 1).
Table 1.
Baseline characteristics according to restless legs syndrome status in 2002 in the Health Professionals Follow-up Studya
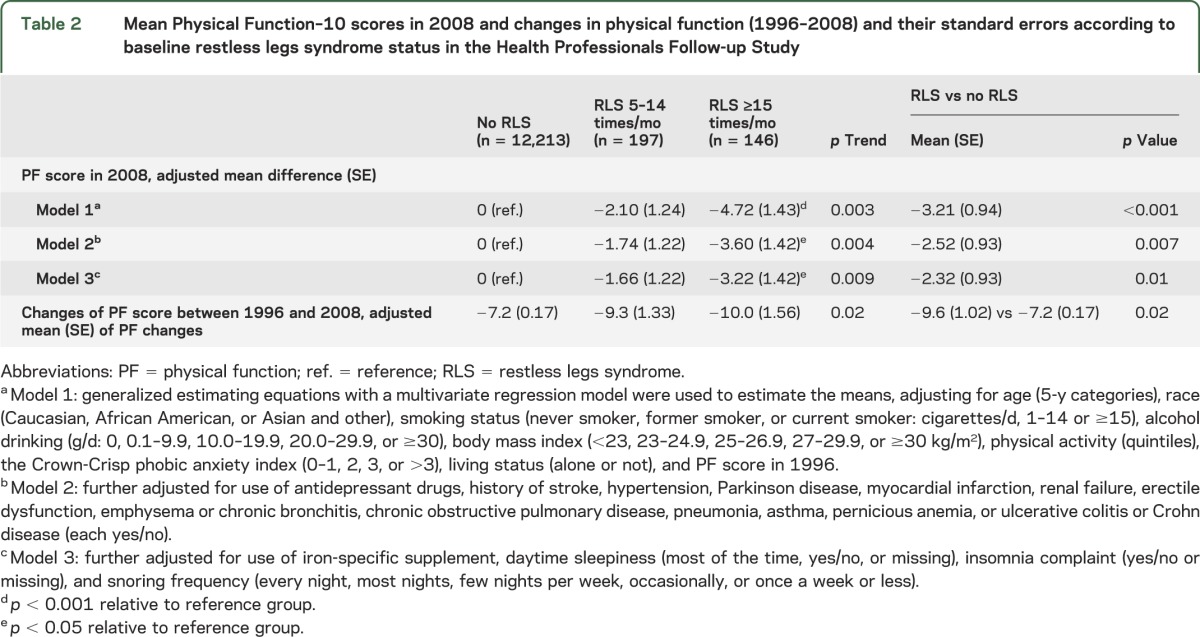
There was a significant trend in decreasing mean PF scores as the frequency of RLS symptoms increases, even after adjusting for potential cofounders such as baseline chronic diseases and other sleep parameters (table 2). Compared to non-RLS participants, RLS participants as one group had significantly lower mean PF scores, after adjusting for controlled covariates. There is a significant trend association between severity of RLS, as assessed by the frequency of symptoms, and decrease in PF score from 1996 to 2008 (table 2).
Table 2.
Mean Physical Function–10 scores in 2008 and changes in physical function (1996–2008) and their standard errors according to baseline restless legs syndrome status in the Health Professionals Follow-up Study
Having daily daytime sleepiness was associated with lower mean PF value than not having the symptom (p < 0.001) (figure 1A). Having insomnia was marginally associated with a lower PR (p = 0.06). In contrast, frequent snoring was not significantly associated with lower mean PF value. The presence of OSA was not asked by the 2008 HPFS questionnaire. Snoring is a surrogate measure for OSA, though the authors are aware that snoring and OSA may not be equivalent. Sleep duration had a trend toward an umbrella-shaped association with PF scores depending on the duration (figure 1B).
Figure 1. Mean Physical Function–10 scores in 2008 according to other sleep parameters in 2004 and sleep duration in the Health Professionals Follow-up Study.
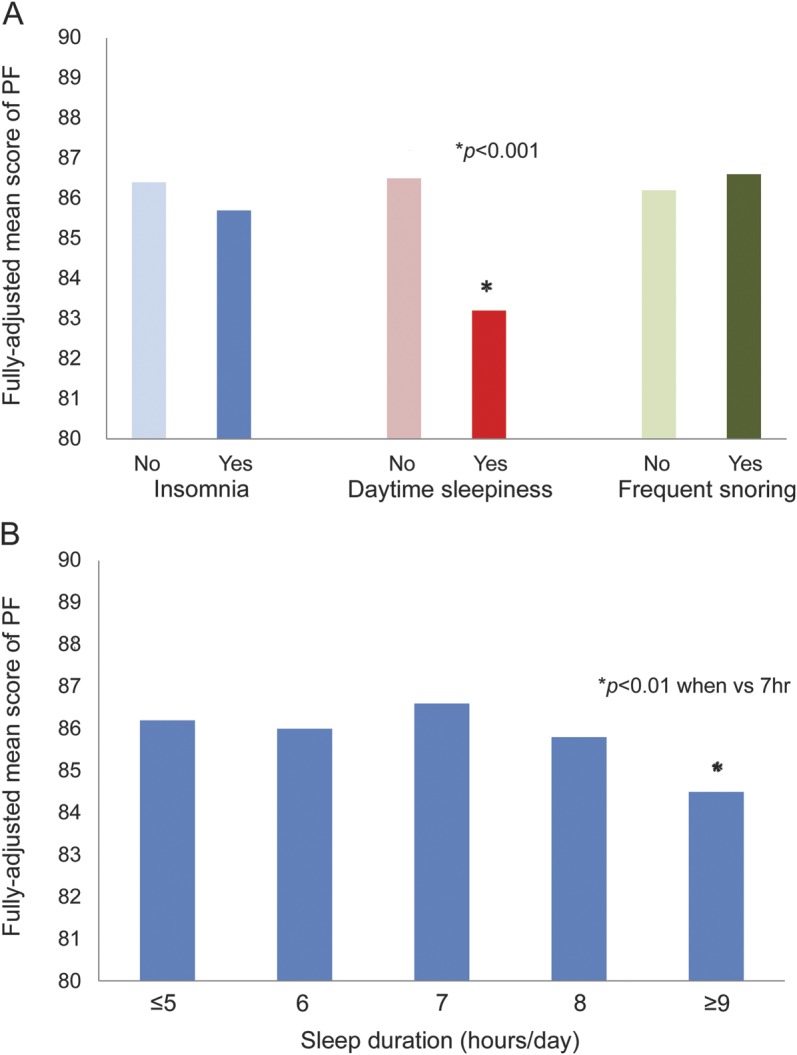
Generalized estimating equations with a multivariate regression model were used to estimate the means, adjusting for age (<60, 60–64, 65–69, 70–74, 75–79, or ≥80 years), race (Caucasian, African American, or Asian and other), smoking status (never smoker, former smoker, or current smoker: cigarettes/d, 1–14 or ≥15), alcohol drinking (g/d: 0, 0.1–9.9, 10.0–19.9, 20.0–29.9, or ≥30), body mass index (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), physical activity (quintiles), the Crown-Crisp phobic anxiety index (0–1, 2, 3, or >3), living status (alone or not), Physical Function (PF)–10 score in 1996, use of antidepressant drugs, history of stroke, hypertension, Parkinson disease, myocardial infarction, renal failure, erectile dysfunction, emphysema or chronic bronchitis, chronic obstructive pulmonary disease, pneumonia, asthma, pernicious anemia, or ulcerative colitis or Crohn disease (each yes/no), and use of iron-specific supplement. (A) Frequent snoring was defined as snoring every night or most nights of the week. Daily daytime sleepiness was defined if the participant answered “most of the time” to the question “How often do you get so sleepy during the day or the evening that you have to take a nap?” Insomnia was defined if the participant answered “most of the time” to 1 of the first 3 questions on sleep patterns described in Methods. (A) *Significant decrease in mean PF score to reference group (p < 0.001). (B) *Significant decrease in mean PF score to reference group of 7 hours of sleep (p < 0.01). PF = physical function.
We also examined the joint effect between RLS and other sleep complaints on PF status (p-interaction 0.3) (figure 2). The highest PF score was seen among participants without RLS and any other sleep complaints, whereas the lowest PF score was seen in the RLS group with other sleep complaints.
Figure 2. Joint effects of restless legs syndrome and other sleep complaints on Physical Function–10 score in 2008.
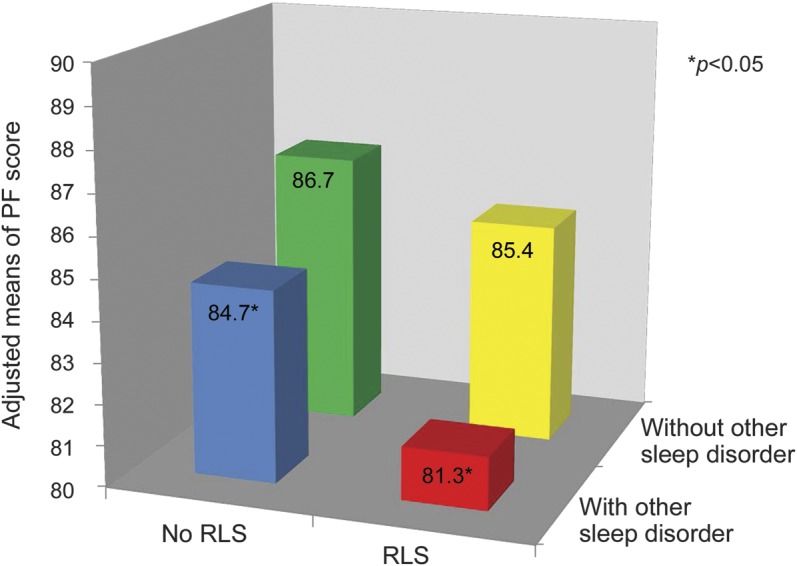
The p for interaction = 0.96. With other sleep complaints was defined if the participant answered at least half of the maximum value of the 4 sleep-related questions described in Methods. The rest of the participants were grouped under “without other sleep complaints.” Generalized estimating equations with a multivariate regression model were used to estimate the physical function (PF) means, adjusting for age (<60, 60–64, 65–69, 70–74, 75–79, or ≥80 years), race (Caucasian, African American, or Asian and other), smoking status (never smoker, former smoker, or current smoker: cigarettes/d, 1–14 or ≥15), alcohol drinking (g/d: 0, 0.1–9.9, 10.0–19.9, 20.0–29.9, or ≥30), body mass index (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), physical activity (quintiles), the Crown-Crisp phobic anxiety index (0–1, 2, 3, or >3), living status (alone or not), PF–10 score in 1996, use of antidepressant drugs, history of stroke, hypertension, Parkinson disease, myocardial infarction, renal failure, erectile dysfunction, emphysema or chronic bronchitis, chronic obstructive pulmonary disease, pneumonia, asthma, pernicious anemia, or ulcerative colitis or Crohn disease (each yes/no), and use of iron-specific supplement. *Significant decrease in mean PF score to reference group who had neither restless legs syndrome (RLS) nor any other sleep complaints (p < 0.05). PF = physical function.
The impact magnitude of the RLS symptoms on PF scores were at least similar to, if not more than, other common conditions such as overweight, smoking, history of MI, hypertension, depression, excessive daytime sleepiness, and insomnia (figure e-2). Of note, the magnitude of PF loss associated with presence of severe RLS vs no RLS was more than that of a 5-year increase of age, having hypertension, or moderate amount of smoking (1–15 cigarettes per day).
We did not find a significant interaction between RLS status and BMI, smoking, and physical activity, in relation to PF-10 score. We observed a marginally significant interaction between RLS status and age (p-interaction = 0.06). The association between RLS and PF score was more pronounced among men aged 65 or older (adjusted mean difference in PF score for RLS vs no RLS = −3.77; p = 0.01), relative to those younger (mean difference = −1.05; p = 0.4). However, it is unclear whether this was due to chance.
We found a similar significant association between RLS and PF status in the sensitivity analyses. The adjusted mean difference in PF score for RLS vs no RLS was −2.34 (p = 0.01), after adjusting for updated statuses of major chronic diseases and the use of antihypertensives, antihistamines, NSAIDs, and acetaminophen in 2008, and −2.69 (p = 0.01) when we excluded men with major chronic diseases (i.e., stroke, MI, obesity, renal failure, cancer, and PD). Finally, we found a stronger association between RLS and PF status after excluding those RLS participants with leg cramps or positional discomfort (adjusted mean difference in PF score for RLS vs no RLS = −3.67, p < 0.001) and iron-deficiency anemia (mean difference = −2.85, p = 0.008).
DISCUSSION
In this large cohort, we found that presence of RLS symptoms and other sleep complains was longitudinally associated with lower future PF in the US elderly population. Even though the overall prevalence of RLS among the general population is high (about 5%–10%), increasing with age, there has been a debate as to whether RLS is a disease warranting timely interventions.6 Hence, understanding the potential long-term impact of RLS on trends in PF and disability is important to address the impact of the disease on QOL and potential health care spending in an aging population.
PF scoring is a marker for ADLs and disability.11,18,22 Previous cross-sectional studies revealed that RLS symptoms were associated with higher disability or lower PF scores.2,5–8,19,20,23–25 Using PF as an indicator of disability, we found that the level of disability over the 6-year period is significantly higher among those participants with baseline RLS symptoms than those without. This difference cannot be accounted for by other covariates such as age, smoking status, obesity, anxiety level, physical activities, iron intake, and presence of other common sleep and nonsleep complaints. The association between RLS and future PF status persisted among the apparently healthy subgroup, who were free of cardiovascular disease, obesity, diabetes, renal failure, and other major conditions. Interestingly, the strength of association between severe RLS symptoms and loss of PF observed in this study is in similar order of magnitude to 5 years of aging and to modifiable risk factors such as overweight, smoking, hypertension, and other common diseases like depression.
A few studies have been done to examine explicitly the burden of RLS on QOL. In a study published in 2004, it was shown that RLS burdened HRQOL across a number of domains when compared to the US general population norm.5 In 2 other cross-sectional studies, RLS-positive women, after controlling for age, sex, and disease comorbidity, had a unique burden of HRQOL compared with the general population norm.7,20 In the RLS epidemiology, symptoms, and treatment (REST) survey, a cross-sectional study conducted in the United States and 5 western European countries, the reduced QOL for RLS was found to be comparable with that experienced by those with type 2 diabetes mellitus and clinical depression, based on the population norm.6,7,24 Another Swedish study showed that the burden of RLS, in terms of PF, on 5,000 women was significant, in a relatively short 9-month follow-up study.19
Our previous work with the same cohort found that RLS is associated with greater mortality. The increased mortality in RLS was more frequently associated with respiratory disease, endocrine disease, nutritional/metabolic disease, and immunologic dysfunction.26 Our findings from the current analysis suggest that poor PF could be one of the pathways to increased mortality for individuals with RLS. This is supported by the observations that cancer patients with declined physical functioning had an increased mortality.27
Our study has a few advantages. Our analyses are results of a large population-based longitudinal study with more than 12,000 participants followed over 6 years. Some prior studies did not measure PF score among individuals without RLS directly and used age- and sex-adjusted population norms as the reference basis for analysis.5,6 We used a baseline PF score measured in the same cohort. Furthermore, unlike most previous studies,5–7,20,24 our model incorporated multiple comorbid conditions of RLS and presence of other sleep complaints in the analyses.
The mechanisms underlying the observed association between RLS alone and future decrease in PF remain unclear. However, recent development in the central pattern generator, a network of spinal neurons, suggests the potential role of the central pattern generator in the development of specific pathophysiologic conditions associated with impaired locomotion or spontaneous locomotor-like movements such as RLS. Spinal cord injuries are known to trigger periodic leg movements during sleep.28 Further cellular and structural properties of skeletal muscle among patients with RLS have been found to have diminished oxygen uptake, which is important for PF.29 Another potential mechanism includes autonomic dysfunction, which has been associated with both disability and RLS.30,31 We previously reported that individuals with RLS had higher risk of erectile dysfunction,32,33 which was also associated with poor PF.34 However, association between RLS and lower PF remained significant after adjustment for erectile dysfunction in the current analysis.
Our findings also suggest that poorer sleep quality can predict higher disability independently of RLS symptoms. Presence of insomnia or excessive daytime sleepiness is each and together associated with lower PF and greater magnitude of disability. Also new in the finding is that sleep duration lasting more than 9 hours per night is associated with decreased PF and more disability, after adjusting for daytime sleepiness. There is also a nonsignificant trend between short sleep duration and worse functional outcome. Our finding is consistent with multiple other findings suggesting that both short and long sleep duration may be associated with higher morbidity and mortality.35,36 The presence of RLS and other sleep complaints can predict future lower PF and higher disability, and the combination of RLS and other sleep complaints have an additive effect on the magnitude of disability. These findings support the hypothesis that there may be an overlapping neurobiological mechanism underlying cerebral regulation of RLS and other sleep complaints.
Several limitations need to be addressed. First, the participants of this study were all male, mostly Caucasian health professionals in the United States. More studies in the general population with different ethnic, education, and cultural background may be needed to make the study results more generalizable. However, given that the prevalence of RLS is less common among men than women, the impact of RLS on disability in the general population may be more pronounced. Second, we relied on self-reported difficulties in physical functioning rather than objective measures of strength or performance, which allowed some degree of misclassification. Likewise, sleep complaints such as insomnia, hypersomnia, and obstructive sleep apneas were measured by self-reported symptoms in the questionnaires and were not confirmed by objective tests. Third, we employed frequent snoring as surrogate measure of OSA. Even though frequent snoring has been shown to correlate closely with OSA in men and has been used as a surrogate of OSA in previous epidemiologic studies,37,38 misclassification is inevitably introduced. Finally, we only had information on iron-deficiency anemia among patients with RLS, rather than the entire cohort. To mitigate this shortcoming, we employed the use of iron-specific supplement as a surrogate marker for iron deficiency. This approach is supported by the observations that among HPFS participants with RLS, those who reported use of iron supplement were 9 times more likely to have iron-deficiency anemia (p < 0.001). We did a sensitivity analysis by excluding those patients with RLS with iron-deficiency anemia, and we generated similar results.
Supplementary Material
GLOSSARY
- ADL
activities of daily living
- BMI
body mass index
- ESRD
end-stage renal disease
- HPFS
Health Professionals Follow-up Study
- HRQOL
health-related quality of life
- MI
myocardial infarction
- NSAID
nonsteroidal anti-inflammatory drug
- OSA
obstructive sleep apnea
- PD
Parkinson disease
- PF
physical function
- PF-10
Physical Function survey of the Short Form–36 Health Survey
- QOL
quality of life
- RLS
restless legs syndrome
- SF-36
Short Form–36 Health Survey
Footnotes
Editorial, page 1198
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Conception and design: C. Zhang, X. Gao. Analysis and interpretation of the data: C. Zhang, Y. Li, X. Gao. Drafting of the article: C. Zhang. Critical revision of the article for important intellectual content: Y. Li, A. Malhotra, Y. Ning, X. Gao. Final approval of the article: C. Zhang, Y. Li, A. Malhotra, Y. Ning, X. Gao. Provision of study materials or patients: X. Gao. Statistical expertise: Y. Li, X. Gao. Collection and assembly of data: Y. Li, X. Gao. Obtaining of funding: X. Gao.
STUDY FUNDING
Supported by NIH/National Institute of Neurological Disorders and Stroke grants of R01 P01 CA055075 and 5R01NS062879-03. None of the sponsors participated in the study design, data collection, analysis, or interpretation.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Broman JE, Mallon L, Hetta J. Restless legs syndrome and its relationship with insomnia symptoms and daytime distress: epidemiological survey in Sweden. Psychiatry Clin Neurosci 2008;62:472–475 [DOI] [PubMed] [Google Scholar]
- 2.Earley CJ, Silber MH. Restless legs syndrome: understanding its consequences and the need for better treatment. Sleep Med 2010;11:807–815 [DOI] [PubMed] [Google Scholar]
- 3.Yang C, Winkelman JW. Clinical and polysomnographic characteristics of high frequency leg movements. J Clin Sleep Med 2010;6:431–438 [PMC free article] [PubMed] [Google Scholar]
- 4.Trenkwalder C, Hogl B, Winkelmann J. Recent advances in the diagnosis, genetics and treatment of restless legs syndrome. J Neurol 2009;256:539–553 [DOI] [PubMed] [Google Scholar]
- 5.Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther 2004;26:925–935 [DOI] [PubMed] [Google Scholar]
- 6.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med 2005;165:1286–1292 [DOI] [PubMed] [Google Scholar]
- 7.Kushida C, Martin M, Nikam P, et al. Burden of restless legs syndrome on health-related quality of life. Qual Life Res 2007;16:617–624 [DOI] [PubMed] [Google Scholar]
- 8.Happe S, Reese JP, Stiasny-Kolster K, et al. Assessing health-related quality of life in patients with restless legs syndrome. Sleep Med 2009;10:295–305 [DOI] [PubMed] [Google Scholar]
- 9.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III: tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40–66 [DOI] [PubMed] [Google Scholar]
- 10.Muller S, Thomas E, Peat G. Derivation and testing of an interval-level score for measuring locomotor disability in epidemiological studies of middle and old age. Qual Life Res 2009;18:1341–1355 [DOI] [PubMed] [Google Scholar]
- 11.Boyle PA, Buchman AS, Wilson RS, Bienias JL, Bennett DA. Physical activity is associated with incident disability in community-based older persons. J Am Geriatr Soc 2007;55:195–201 [DOI] [PubMed] [Google Scholar]
- 12.Hart DL, Wright BD. Development of an index of physical functional health status in rehabilitation. Arch Phys Med Rehabil 2002;83:655–665 [DOI] [PubMed] [Google Scholar]
- 13.Walters AS. Toward a better definition of the restless legs syndrome: The International Restless Legs Syndrome Study Group. Mov Disord 1995;10:634–642 [DOI] [PubMed] [Google Scholar]
- 14.Allen R, Picchietti D, Hening W, Trenkwalder C, Walters AS, Montplaisir J; Restless Legs Syndrome Diagnosis and Epidemiology Workshop at the National Institutes of Health; International Restless Legs Syndrome Study Group. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology: a report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 2003;4:101–119 [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Schwarzschild MA, Wang H, Ascherio A. Obesity and restless legs syndrome in men and women. Neurology 2009;72:1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen R, Burchell BJ, MacDonald B, Hening W, Earley C. Validation of the self-completed Cambridge-Hopkins questionnaire (CH-RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey. Sleep Med 2009;10:1097–1100 [DOI] [PubMed] [Google Scholar]
- 17.Haley SM, McHorney CA, Ware JE., Jr Evaluation of the MOS SF-36 physical functioning scale (PF-10): I: unidimensionality and reproducibility of the Rasch item scale. J Clin Epidemiol 1994;47:671–684 [DOI] [PubMed] [Google Scholar]
- 18.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988-2004. JAMA 2007;298:2020–2027 [DOI] [PubMed] [Google Scholar]
- 19.Wesstrom J, Nilsson S, Sundstrom-Poromaa I, Ulfberg J. Health-related quality of life and restless legs syndrome among women in Sweden. Psychiatry Clin Neurosci 2010;64:574–579 [DOI] [PubMed] [Google Scholar]
- 20.Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med 2004;164:196–202 [DOI] [PubMed] [Google Scholar]
- 21.Zucconi M, Ferini-Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med 2004;5:293–299 [DOI] [PubMed] [Google Scholar]
- 22.Wolinsky FD, Bentler SE, Hockenberry J, et al. Long-term declines in ADLs, IADLs, and mobility among older Medicare beneficiaries. BMC Geriatr 2011;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hening W, Allen RP, Tenzer P, Winkelman JW. Restless legs syndrome: demographics, presentation, and differential diagnosis. Geriatrics 2007;62:26–29 [PubMed] [Google Scholar]
- 24.McCrink L, Allen RP, Wolowacz S, Sherrill B, Connolly M, Kirsch J. Predictors of health-related quality of life in sufferers with restless legs syndrome: a multi-national study. Sleep Med 2007;8:73–83 [DOI] [PubMed] [Google Scholar]
- 25.Winkelman JW, Redline S, Baldwin CM, Resnick HE, Newman AB, Gottlieb DJ. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep 2009;32:772–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Wang W, Winkelman JW, Malhotra A, Ma J, Gao X. Prospective study of restless legs syndrome and mortality among men. Neurology 2013;81:52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sehl M, Lu X, Silliman R, Ganz PA. Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J Cancer Surv 2013;7:20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Telles S, Alves R, Chadi G. Spinal cord injury as a trigger to develop periodic leg movements during sleep: an evolutionary perspective. Arq Neuropsiquiatr 2012;70:880–884 [DOI] [PubMed] [Google Scholar]
- 29.Larsson B, Kadi F, Ulfberg J, Aulin K. Skeletal muscle morphology in patients with restless legs syndrome. Eur Neurol 2007;58:133–137 [DOI] [PubMed] [Google Scholar]
- 30.Flachenecker P, Reiners K, Krauser M, Wolf A, Toyka KV. Autonomic dysfunction in multiple sclerosis is related to disease activity and progression of disability. Mult Scler 2001;7:327–334 [DOI] [PubMed] [Google Scholar]
- 31.Cikrikcioglu MA, Hursitoglu M, Erkal H, et al. Oxidative stress and autonomic nervous system functions in restless legs syndrome. Eur J Clin Invest 2011;41:734–742 [DOI] [PubMed] [Google Scholar]
- 32.Gao X, Chen H, Schwarzschild MA, et al. Erectile function and risk of Parkinson's disease. Am J Epidemiol 2007;166:1446–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Batool-Anwar S, Kim S, Rimm EB, Ascherio A, Gao X. Prospective study of restless legs syndrome and risk of erectile dysfunction. Am J Epidemiol 2013;177:1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujisawa M, Sawada K, Okada H, Arakawa S, Saito S, Kamidono S. Evaluation of health-related quality of life in patients treated for erectile dysfunction with Viagra (sildenafil citrate) using SF-36 score. Arch Androl 2002;48:15–21 [DOI] [PubMed] [Google Scholar]
- 35.Hoevenaar-Blom M, Spijkerman A, Kromhout D, van den Berg J, Verschuren W. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep 2011;34:1487–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu L, Sautter J, Liu Y, Gu D. Age and gender differences in linkages of sleep with subsequent mortality and health among very old Chinese. Sleep Med 2011;12:1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010;33:585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bliwise DL, Nekich JC, Dement WC. Relative validity of self-reported snoring as a symptom of sleep apnea in a sleep clinic population. Chest 1991;99:600–608 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


