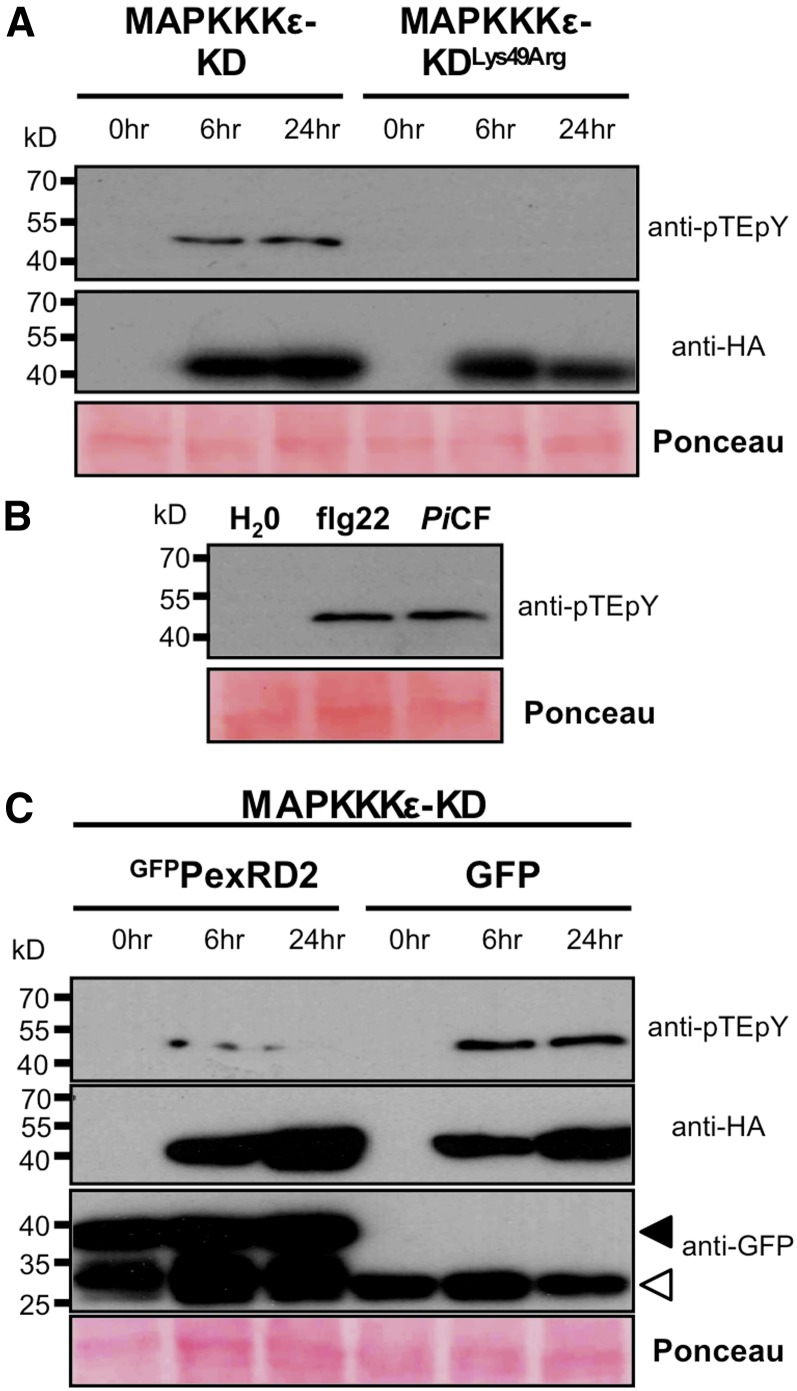
Figure 8.
MAPKKKε-Mediated Activation of MAPKs Is Suppressed by PexRD2.
(A) Immunoblot analysis shows that overexpression of SlMAPKKKε-KD causes activation of an endogenous MAPK in N. benthamiana. Overexpression of the kinase-inactive mutant (MAPKKKε-KDLys49Arg) did not phosphorylate MAPK. Protein levels of both kinases at 6 and 24 h post-β-estradiol treatment were confirmed using an anti-HA antibody (also in [C]).
(B) Treatment of N. benthamiana leaf disks with peptide corresponding to the epitope of the bacterial PAMP flagellin, flg22 (100 nM), or 100-fold diluted P. infestans 88069 culture filtrate (PiCF) results in activation of MAPK.
(C) Coexpression of GFPPexRD2, but not GFP, suppresses the activation of MAPK mediated by SlMAPKKKε-KD. Expression of GFPPexRD2 and SlMAPKKKε-KD was confirmed using anti-GFP and anti-HA antibodies, respectively. Black arrow indicates expected size of the GFPPexRD2 fusion protein and the white arrow free GFP. Similar results were observed in three independent experiments. In all cases, protein loading is confirmed by Ponceau staining.