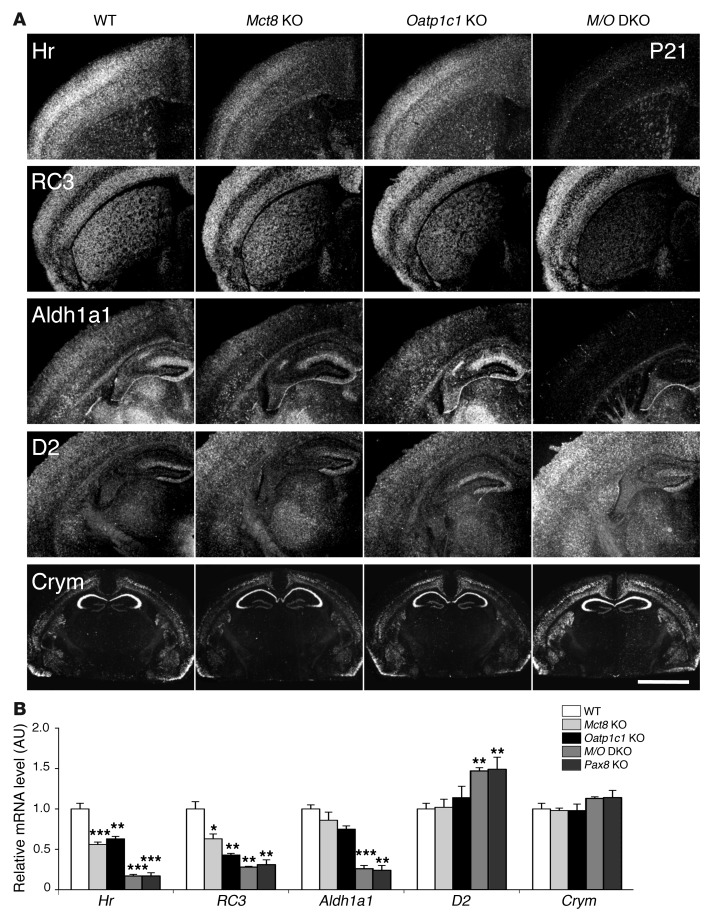
Figure 5. Consequences of combined MCT8 and OATP1C1 deficiency on T3 target gene expression in the CNS.
(A) ISH studies performed using brain sections at P21 (n = 4 per genotype) revealed for Mct8/Oatp1c1 DKO mice a strong downregulation in the signal intensities for Hr, RC3, and Aldh1a1, all known to be positively regulated by T3. Hr expression was most prominently decreased throughout the cerebral cortex, whereas RC3 expression was highly reduced in neurons of the striatum. In cortical areas, Aldh1a1-specific ISH signals were specifically reduced in astrocytes, but relatively unaltered in capillary endothelial cells. Darkfield autoradiograms also illustrated upregulation of D2 in glial cells. Interestingly, mRNA expression of Crym, an intracellular TH binding protein, was elevated specifically in the cortex and striatum. Scale bar: 600 μm (Hr, RC3, Aldh1a1, and D2); 3 mm (Crym). (B) qPCR analysis was performed using forebrain homogenates from P21 animals (n = 4–5 per genotype). Athyroid Pax8 KO mice were included and showed changes in forebrain gene expression similar to those of Mct8/Oatp1c1 DKO mice. These findings again pointed to a TH-deprived CNS in the absence of MCT8 and OATP1C1. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT.