Figure 3.
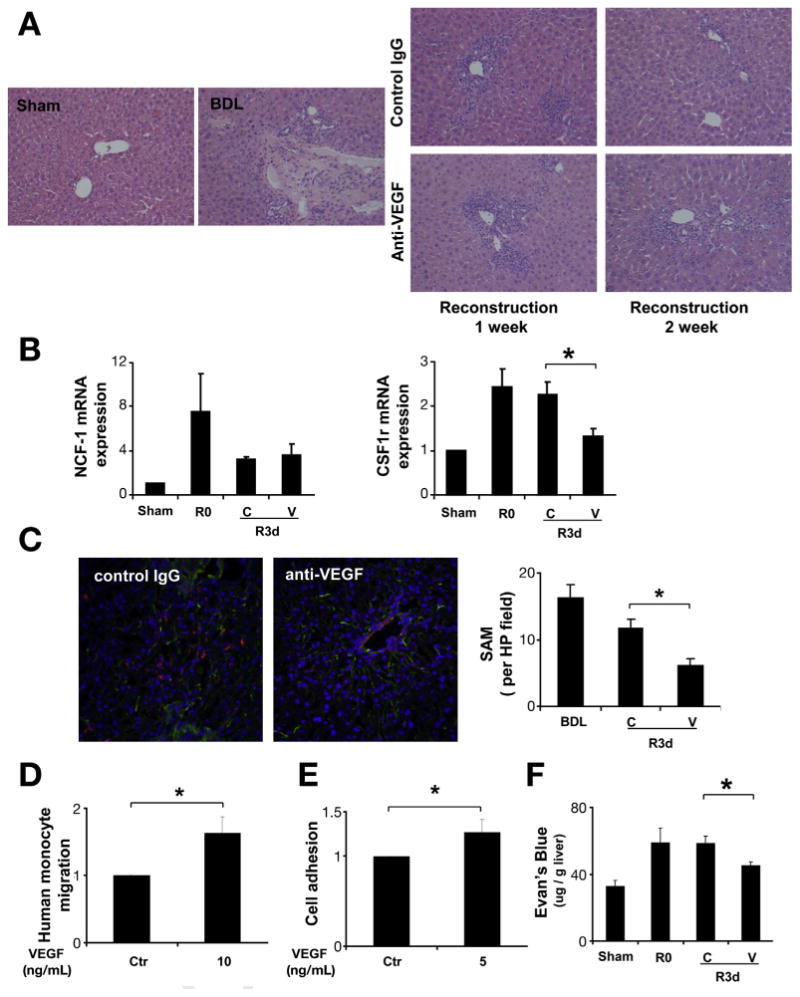
Anti-VEGF antibody decreased SAM during fibrosis resolution. C57BL/6 mice received VEGF-neutralizing antibody or control antibody (IP ×2/week for 1 or 2 weeks), after 2 weeks of BDL followed by CJ. One week and 2 weeks after CJ, livers were harvested and subjected to analysis. H&E staining (200×) showed significant inflammatory cell infiltration during scar resolution (A). Quantitative reverse transcription polymerase chain reaction from whole-liver mRNA (B) was performed to evaluate the expression level of the macrophage marker CSF1R, and the neutrophil marker NCF1. CSF1R mRNA levels were decreased after neutralizing VEGF antibody treatment during fibrosis resolution, and no change was seen in NCF1 mRNA levels. Immunofluorescence for collagen I (green) and macrophage marker F4–80 (red) were used to identify SAM in frozen section as describe in Materials and Methods (200×). VEGF neutralization significantly decreased SAM during liver fibrosis regression at day 3 (C). Cell migration was measured 3 hours later by Boyden chamber. VEGF (10 ng/mL) stimulated monocyte migration showed in (D). To evaluate the effect of VEGF on monocyte adhesion, HUVEC cell was treated with VEGF for 12 hours before co-culture with florescent dye labeled monocyte for an additional 1 hour. Cells were washed and fluorescence was measured to determine cell adhesion (E). Mice received one dose of VEGF-neutralizing antibody or control antibody after BDL + CJ. Three days later, mice received intravenous Evan's blue dye 30 minutes before sacrifice. Tissue Evans blue content in the liver (μg Evans blue/g liver tissue) was measured to assess vascular permeability (F). (R0: BDL 2 weeks without CJ; R3d: BDL 2 weeks plus CJ for 3 days; C: control IgG; V: anti-VEGF antibody; *P < .05).
