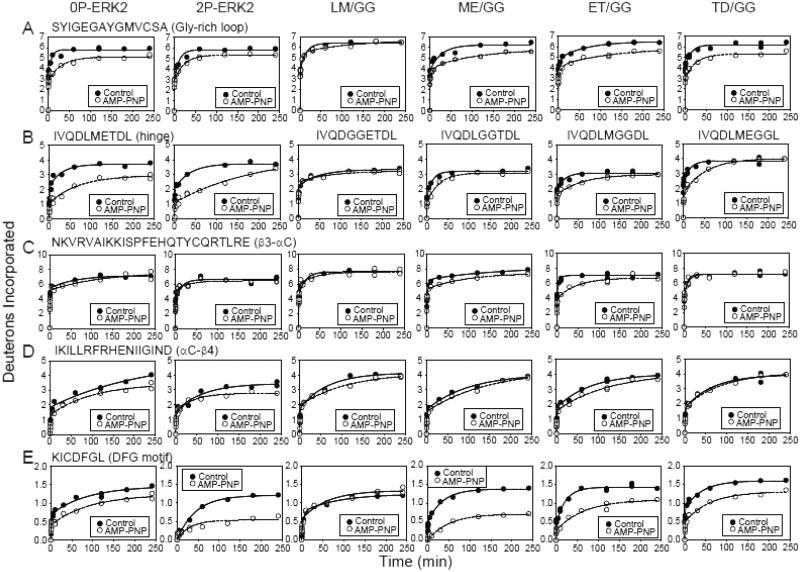
Fig. 3. HX protection in ERK2 upon AMP-PNP binding.
HX time courses comparing WT and mutant ERK2 in the presence (○) or absence (●) of AMP-PNP. All mutants except LM/GG showed HX protection in regions previously found to interact with nucleotide. (A) Peptide SYIGEGAYGMVCSA comprises the Gly-rich loop, where AMP-PNP binding protects against HX by >1 Da. (B) Peptide IVQDLMETDL contains the hinge region where AMP-PNP binding yields lower HX protection in the hinge mutants than WT-ERK2. (C,D) Peptides NKVRVAIKKISPFEHQTYCQRTLRE and IKILLRFRHENIIGIND comprise the β3-αC-β4 region, which coordinates Mg2+-ATP. HX protection occurs within αC-β4 in WT-ERK2 and within β3-αC in mutants ME/GG- and ET/GG-ERK2. (E) Peptide KICDFGL corresponds to the DFG motif in the C-terminal domain. HX protection by nucleotide binding is greater in 2P-ERK2 and in mutants ME/GG- and ET-GG-ERK2 compared to 0P-ERK2. The results suggest that ME/GG, and to a lesser extent ET/GG, promote interdomain interactions in ERK2, even without activation loop phosphorylation.