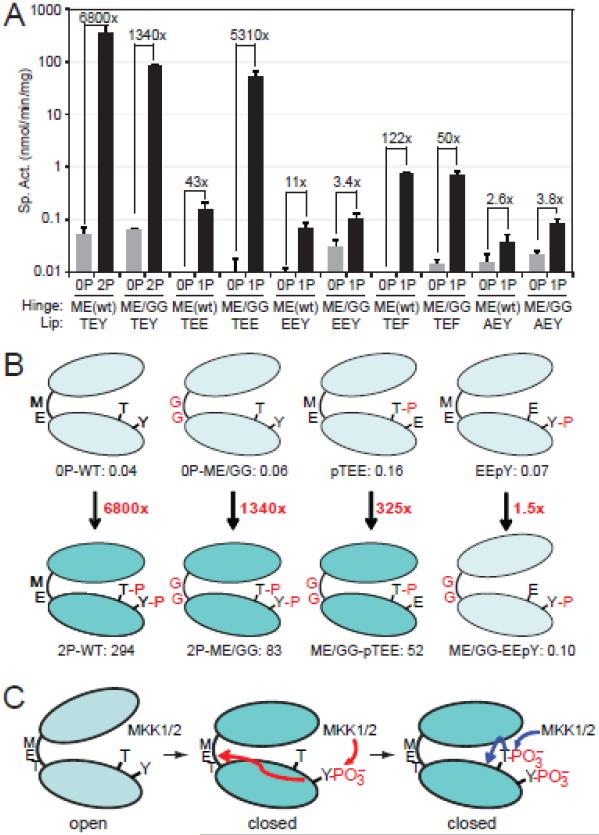
Fig. 5. Hinge mutations activate ERK2 monophosphorylated at Thr183.
(A) Specific activities of WT- and ME/GG-ERK2, harboring activation loop mutations TEE, EEY, TEF, and AEY. Mutants were assayed in their unphosphorylated (−) forms or following phosphorylation by active MKK1 (+). Bars show average ± s.d. of triplicate measurements. Significant kinase activation is observed in ME/GG-pTEE-ERK2, compared to monophosphorylated pTEE-ERK2 or unphosphorylated ME/GG-ERK2. Data for other mutant forms are shown in Fig. S3. (B) Summary showing ERK2 activation by ME/GG in combination with pTEE but not EEpY. (C) Model for ERK2 activation by regulated dynamics. Prior to phosphorylation, ERK2 is constrained from domain movement and adopts an inactive solution conformation. Phosphorylation at Tyr185 by MKK1 enhances backbone flexibility in the hinge region, allowing domain movement and adoption of interactions needed to optimize the catalytic site. Phosphorylation at Thr183 stabilizes the activation loop conformation and modulates other events needed for catalytic site configuration.