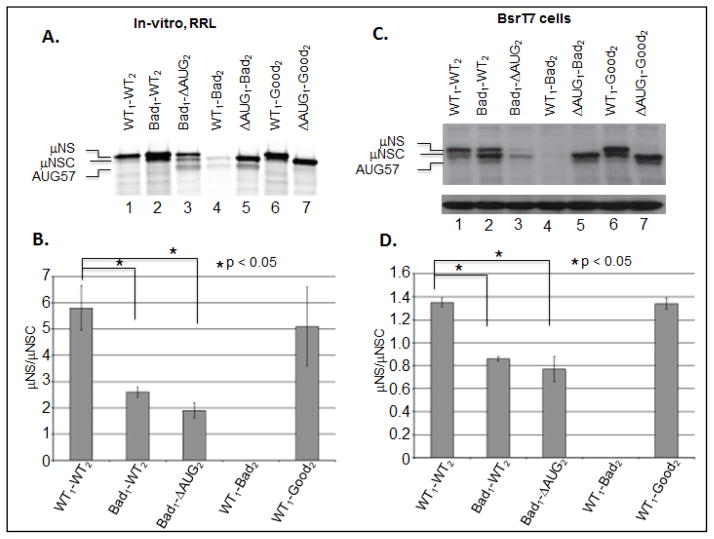
Figure 4. Altering the Kozak context of either the upstream or downstream initiation sites of the MRV M3 mRNA alters the μNS/μNSC ratio.
A) In-vitro RRL translation reactions were programmed with 2 μg of WT, Kozak mutant mRNAs. Reactions were incubated with [35S]methionine at 30°C for 90 minutes. Reactions were stopped by addition of 2X Laemmli sample buffer and labeled proteins were separated by 8% SDS-PAGE. Labeled proteins were visualized by phosphorimaging. B) Radiolabeled μNS and μNSC proteins, visualized by phosphorimaging, were quantified using ImageQuant 7.0 software. μNS/μNSC rations were determined for each mRNA construct (ΔAUG1 constructs were excluded from analysis). μNS/μNSC rations are presented as the arithmetic mean ± SD of three independent assays in which samples were analyzed in triplicate. Significance was determined by Student’s t-test where P<0.05 was considered significant. C) In-vitro transcribed WT and mutant construct mRNAs were transfected into BsrT7 cells. AT 24 h p.t. cells were lysed and μNS, μNSC and GAPDH proteins were detected Immunoblotting. D) μNS and μNSC protein concentrations were determined by densitometry, and μNS/μNSC ratios were calculated and plotted. μNS/μNSC rations are presented as the arithmetic mean ± SD of three independent assays with different preparations of RNA in which samples were analyzed in triplicate. Significance was determined by Student’s t-test where P<0.05 was considered significant.