Abstract
Nitrite has been postulated to provide a reservoir for conversion to nitric oxide (NO), especially in tissues with reduced oxygen levels as in the fetus. Nitrite would thus provide local vasodilatation and restore a balance between oxygen supply and need, a putative mechanism of importance especially in the brain. The current experiments test the hypothesis that exogenous nitrite acts as a vasodilator in the cephalic vasculature of the intact, near term fetal sheep. Fetuses were first instrumented to measure arterial blood pressure and carotid artery blood flow and then studied 4–5 days later while in utero without anaesthesia. Initially l-nitro-arginine (LNNA) was given to block endogenous NO production. Carotid resistance to flow increased 2-fold from 0.54 ± 0.01 (SEM) to 1.20 ± 0.08 mmHg min ml−1 (in 13 fetuses, P < 0.001), indicating NO tonically reduces cerebral vascular tone. Sodium nitrite (or saline as control) was then infused in increasing step-doses from 0.01 to 33 μm in half-log increments over a period of 2 h. Carotid artery pressure, blood flow and vascular resistance did not change compared to fetuses receiving saline, even at plasma nitrite concentrations two orders of magnitude above the physiological range. The results indicate that while cephalic vascular tone is controlled by endogenous nitric oxide synthase activity, exogenously administered nitrite is not a vasodilator at physiological concentrations in the vasculature served by the carotid artery of fetal sheep.
Introduction
Evidence has been accumulating that nitrite is not merely an inert end-product of NO metabolism but that it may be converted back into NO preferentially under hypoxic and ischaemic conditions by a number of biochemical pathways (see review by Lundberg & Weitzberg, 2010). In the blood, this conversion can occur by a reaction between nitrite and deoxygenated haem centres of haemoglobin as follows:
| Reaction 1 |
This reaction, which is sensitive to haemoglobin oxygen saturation, has been proposed to produce vasodilating quantities of NO, thereby matching oxygen supply with oxygen requirement (Cosby et al. 2003; Huang et al. 2005; Gladwin et al. 2006).
There are several reasons to expect that Reaction 1 would play a greater role in mediating vasodilatation in the fetus than in the adult. First, the rate of Reaction 1 is markedly increased when haemoglobin is near 50% saturated with O2 (Huang et al. 2005; Crawford et al. 2006; Blood & Power, 2007), and fetal arterial haemoglobin is closer to this level of oxygenation than the adult due to the lower overall O2 tensions of the fetus. Secondly, fetal haemoglobin reduces nitrite to NO at a rate 2-to 3-fold faster than adult haemoglobin (Blood et al. 2009) and the more rapid rate is observed while taking account of differences in haemoglobin concentration, pH and oxygen saturation. Lastly, Reaction 1 is favoured by lower pH and increased haemoglobin concentration, both of which are normally true of fetal blood relative to maternal blood in both humans and sheep (Longo & Pearce, 1998; Rothstein & Longo, 1998).
We therefore hypothesized that nitrite would play a particularly prominent role in modulation of the resistance of fetal vessels. Here we describe experiments to test this hypothesis by administering infusions of sodium nitrite into the carotid artery of chronically instrumented fetal sheep while measuring carotid pressure and flow, and calculating vascular resistance. Infusions were performed in the presence and absence of l-nitro-arginine (LNNA) to block endogenous NO production by nitric oxide synthases.
Methods
The experimental procedures were pre-approved by the Loma Linda University Institutional Animal Care and Use Committee and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgical instrumentation
After induction with intravenous thiopental (10 mg kg−1, i.v.), pregnant ewes (123–130 days gestation) were intubated and anaesthesia was then maintained with 2% isoflurane in oxygen. Through a midline abdominal incision, the uterus was opened and the fetus exposed. A catheter was introduced into the right lingual artery and its tip advanced to the common carotid artery without obstructing carotid flow. This catheter was used to infuse nitrite directly into the carotid blood stream. A 3R transonic flow probe (3 mm, R series; Transonic Systems Inc., Ithaca, NY, USA) was placed around the right common carotid artery for measurement of unilateral carotid flow (Gratton et al. 1996). Brachial artery and external jugular vein catheters were placed for arterial and venous blood sampling. Arterial blood pressure (Cobe, Lakewood, CO, USA) and heart rate were monitored via a femoral artery catheter. Additional catheters were introduced into one femoral vein and the amniotic cavity for drug administration. The latter was also used for measurement of intrauterine pressure. The vascular catheters and electrical leads from the flow probe were then passed through the uterine and maternal abdominal walls and secured in a pouch on the ewe's flank.
Recovery time from surgery was 4–5 days prior to the experiment. Daily, the ewes were given intramuscular penicillin (900,000 units), and the fetuses were given intra-amniotic ampicillin (500 mg) and gentamicin (40 mg). Fetal condition was monitored daily with arterial blood gases. At the conclusion of the experiments, the ewes and fetuses were euthanized with an intravenous bolus of Euthasol (20 ml, Western Medical Supply, Arcadia, CA, USA.
Nitrite infusion
Experiments were carried out at 128–135 days of gestation (term is 145 days). After 30 min of baseline measurements (120 min before nitrite infusion), LNNA was given to the fetus as a 45 mg kg−1 intravenous bolus (100 mg total for assumed 2.5 kg fetal weight). Sixty minutes later, LNNA was given to the ewe at the same dosage. This drug was given to block endogenous generation of NO by nitric oxide synthases; its plasma elimination half-life is 20 h after an intravenous bolus injection in rats (Tabrizi-Fard & Fung, 1996).
Sixty minutes after the maternal LNNA was given, the fetal sheep were arbitrarily assigned to receive either saline (LNNA + saline control group) or sodium nitrite dissolved in saline (LNNA + nitrite group). Solutions were infused into the right common carotid artery of the fetus at an infusion rate of 0.67 ml min−1 for a total of 120 min. The concentration of nitrite being infused was increased by half-log increments every 15 min (Fig. 1). Based on an estimated carotid blood flow of 50 ml min−1, which is in the range typical for this age of sheep fetus (Rybakowski et al. 2000), the nitrite infusions were predicted to increase carotid blood nitrite concentrations in half-log increments from 0.020 to 66.6 μm, the maximal concentration being approximately 20-fold higher than physiological concentrations (Blood et al. 2009). Arterial and venous blood samples for blood gas analysis and haemoximetry (ABL-800, Radiometer, Copenhagen, Denmark) and measurement of nitrite concentrations were drawn every 30 min until completion of nitrite or saline infusions, and 15 and 45 min afterward.
Figure 1. Protocol of nitrite infusion into carotid artery of fetal sheep.
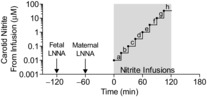
LNNA was given intravenously to the fetus (−120 min) and ewe (−60 min) and nitrite or saline infusions into a fetal carotid artery were begun at time 0. Nitrite infusion increased systemic fetal blood nitrite concentrations from ∼10 nm (infusion ‘a’) to 33,333 nm (infusion ‘h’) in half-log incremental steps every 15 min. Physiological concentrations were estimated to occur during steps ‘c’ to ‘e’. Concentrations of nitrite in the carotid artery were calculated based on a unilateral carotid flow of 50 ml min−1.
To address the possibility that LNNA may have attenuated responses to nitrite infusion, an additional group of fetuses were studied with the same nitrite infusion protocol but without prior exposure to LNNA (no LNNA + nitrite group). In addition, to assess the capacity of the cephalic vasculature to respond to NO, three of these fetuses received an intra-carotid bolus of the NO donor 1-(hydroxy-NNO-azoxy)-l-proline (PROLI-NO) as 1 ml of a 0.5 mm solution infused over 30 s.
Nitrite assay
Immediately after collection, 0.4 ml of whole blood was added to 0.1 ml of nitrite preservation solution containing 0.8 m ferricyanide, 0.1 m N-ethylmaleimide, and 10% Nonidet P-40. The sample was vortexed, immersed in liquid nitrogen and then stored at −70°C until assay. Nitrite concentrations were determined using tri-iodide chemiluminescence as described by Pelletier et al. (2006). It should be noted that the method detects and quantifies the combined concentrations of nitrite, S-nitrosothiols, N-nitrotyrosines and iron-nitrosyl species (Feelisch et al. 2002; Wang et al. 2006) above 10 nm and has a precision of ±5 nm. In our hands, removal of nitrite with acid sulfanilamide, as previously described (Feelisch et al. 2002; Wang et al. 2006), eliminates >97% of the signal in samples collected with the preservation solution described above, and thus we designate these measurements here as nitrite. The assay does not detect nitrate.
Data analysis
Arterial blood pressure, carotid blood flow and amniotic fluid pressure were recorded continuously throughout each experiment. After each experiment, amniotic fluid pressure was subtracted from the fetal arterial pressure signal. Arterial pressure and carotid flow were then re-sampled as 15 min averages. Resistance to flow in the vasculature supplied by the carotid artery was calculated as the ratio of mean arterial blood pressure to carotid flow rate. Data are presented as mean ± SEM. One-way ANOVA with repeated measures and Dunnett's multiple comparison test were used to determine significance of changes over time. Differences between groups were detected using two-way ANOVA followed by Bonferroni post hoc analysis. Statistical analyses were performed using Prism 5 for Mac OS X (Graphpad Software, La Jolla, CA, USA).
Results
Experiments were completed in a total of 17 fetal sheep. Among these, seven received LNNA + nitrite, six received LNNA + saline, and four received nitrite only (no LNNA). The gestational ages were comparable in the three groups, 131 ± 1, 132 ± 1 and 131 ± 1 days, respectively.
Blood gases and haemoximetry
Arterial pH, 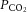 ,
, 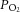 and oxyhaemoglobin saturation did not differ among the study groups during the baseline period, after LNNA infusion, or during nitrite or saline infusion (Fig. 2). In the two groups that received LNNA, a mild respiratory acidosis developed over the course of the experiment, while arterial
and oxyhaemoglobin saturation did not differ among the study groups during the baseline period, after LNNA infusion, or during nitrite or saline infusion (Fig. 2). In the two groups that received LNNA, a mild respiratory acidosis developed over the course of the experiment, while arterial 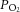 and oxyhaemoglobin saturations remained unchanged (Fig. 2). Baseline methaemoglobin concentrations were similar in the three groups and varied in the nitrite-treated groups from 1.1 ± 0.3% at baseline to a peak value of 1.4 ± 0.2% during the highest rate of nitrite infusion (P = 0.92).
and oxyhaemoglobin saturations remained unchanged (Fig. 2). Baseline methaemoglobin concentrations were similar in the three groups and varied in the nitrite-treated groups from 1.1 ± 0.3% at baseline to a peak value of 1.4 ± 0.2% during the highest rate of nitrite infusion (P = 0.92).
Figure 2. Arterial blood gas responses to infusion of nitrite or saline in fetal sheep.
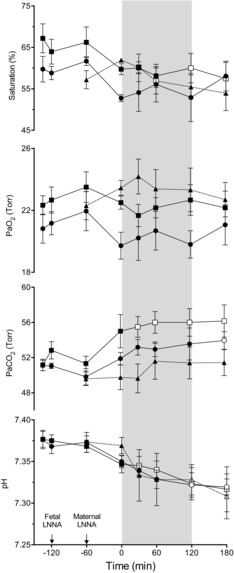
Results are shown for fetuses that received LNNA + saline (squares, n = 6), LNNA + nitrite (circles, n = 7), and saline + nitrite (triangles, n = 4). Open symbols denote values significantly different from baseline values for that group (1-way ANOVA with Bonferroni post hoc analysis). Differences between study groups are not significant (2-way ANOVA). Shaded area shows time period of increasing nitrite dosage.
Response to LNNA
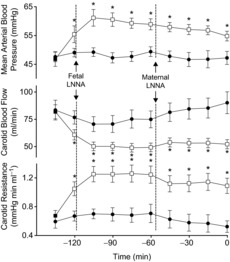
Baseline arterial nitrite concentrations (Fig. 3), blood pressure, heart rate, carotid flow and carotid resistance to flow (Fig. 4) were comparable in the two study groups receiving LNNA. After LNNA infusion, blood pressure increased (to 64 ± 5 and 57 ± 2 mmHg in the LNNA + nitrite and LNNA + saline groups, respectively, P < 0.001 for both groups) as arterial nitrite concentration decreased comparably in the two groups. Similarly, heart rate, carotid flow and the resistance to carotid flow changed comparably in the two groups receiving LNNA. These variables were not significantly changed further after LNNA was given to the ewes.
Figure 3. Arterial nitrite concentrations.
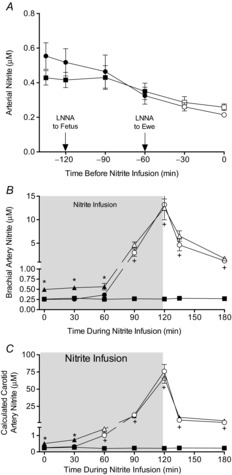
A, brachial artery nitrite concentrations in response to LNNA infusion. B, nitrite concentrations during nitrite infusion in blood sampled from the brachial artery. C, calculated nitrite concentrations in the carotid artery downstream of the site of nitrite infusion, with values based on carotid blood flow, rate of nitrite infusion, and concentration of nitrite measured in the brachial arterial blood. Note that nitrite levels range from less than initial baseline to 100-fold higher than normal at the maximal infusion rate. Squares represent fetuses that received LNNA + saline (n = 6), circles represent fetuses that received LNNA + nitrite (n = 7), and triangles represent the fetuses that received saline + nitrite (n = 4). In A, open symbols denote time points that were significantly different from the −135 min time point. In B and C, open symbols denote time points significantly different from the 0 min time point (1-way ANOVA with Bonferroni post hoc analysis). (+P < 0.05 for comparison between LNNA + nitrite and LNNA + saline groups. *P < 0.05 for comparison between the saline + nitrite and LNNA + nitrite groups (2-way ANOVA with Bonferroni post hoc analysis).
Figure 4. Changes in mean arterial blood pressure, heart rate, carotid blood flow and carotid resistance in response to LNNA bolus followed by infusion of saline or nitrite in stepwise increasing concentrations during the shaded area.
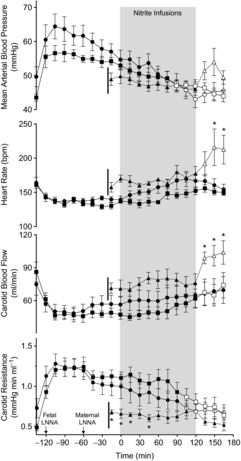
Squares represent fetuses receiving LNNA + saline (n = 6), circles represent fetuses receiving LNNA + nitrite (n = 7), and triangles represent fetuses receiving saline + nitrite (n = 4). Open symbols represent time points that are significantly different from the pre-nitrite infusion values measured at time 0 (1-way ANOVA, Bonferroni post hoc test). LNNA bolus to the fetuses resulted in significant increases in mean arterial blood pressure and carotid vascular resistance to flow, and decreases in heart rate (beats min–1) and carotid blood flow (1-way ANOVA). There were no significant differences between the LNNA + nitrite and LNNA + saline groups. *P < 0.05 for comparison between the LNNA + nitrite and the saline + nitrite groups (2-way ANOVA with Bonferroni post hoc test).
Effect of nitrite infusion on blood nitrite concentrations
Systemic nitrite concentrations had increased significantly to 3.0 ± 0.2 μm when the nitrite infusion rate was 30 μg min−1 (step ‘f’ in Fig. 1), demonstrating recirculation of the carotid nitrite infusion throughout the body (Fig. 3). Systemic nitrite concentrations reached peak levels of 13.2 ± 1.2 μm at the end of the infusion period, approximately 50-fold higher than the post-LNNA baseline, and 20-to 30-fold above physiological concentrations. Carotid nitrite concentrations, calculated based on the rates of nitrite infusion and measured carotid blood flow, became significantly elevated above baseline levels when the infusion rate was at 3 μg min−1 (step ‘d’ in Fig. 1) or higher, peaking at 91 ± 18 μm during the highest nitrite infusion rate (P < 0.001). Brachial artery nitrite concentrations did not change in fetuses given saline, remaining in the range of 0.20–0.30 μm.
Cardiovascular response to nitrite infusion
Carotid flow decreased and resistance to flow increased from baseline after infusion of fetal LNNA (Fig. 4). There were no measurable differences between the nitrite and control groups in responses of arterial blood pressure (P = 0.28), heart rate (P = 0.31), carotid blood flow (P = 0.59), or carotid resistance to flow (P = 0.60). Although there was significant increase in arterial 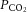 (
(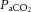 ) in the fetuses receiving saline, the
) in the fetuses receiving saline, the 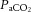 was still within physiological range making this an unlikely cause of vasodilatation in the control fetuses. In fetuses receiving nitrite without LNNA blockade, there were no significant changes in mean arterial blood pressure, heart rate, carotid blood flow, or carotid resistance to flow during the nitrite infusion. In this group blood pressure, heart rate and carotid flow increased significantly after discontinuation of the nitrite infusion, with no significant change in carotid resistance to flow (Fig. 4). In addition, infusion of PROLI-NO into the carotid artery of three of these fetuses resulted in a >50% decrease in carotid vascular resistance (Fig. 5), indicating that the vasculature was still capable of responding to NO.
was still within physiological range making this an unlikely cause of vasodilatation in the control fetuses. In fetuses receiving nitrite without LNNA blockade, there were no significant changes in mean arterial blood pressure, heart rate, carotid blood flow, or carotid resistance to flow during the nitrite infusion. In this group blood pressure, heart rate and carotid flow increased significantly after discontinuation of the nitrite infusion, with no significant change in carotid resistance to flow (Fig. 4). In addition, infusion of PROLI-NO into the carotid artery of three of these fetuses resulted in a >50% decrease in carotid vascular resistance (Fig. 5), indicating that the vasculature was still capable of responding to NO.
Figure 5. Effect of intra-carotid infusion of an NO donor on carotid vascular resistance.
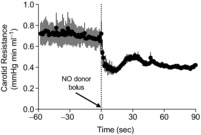
Three of the fetuses that received nitrite but no LNNA were given a bolus of PROLI-NO into the carotid artery at time 0, resulting in a significant decrease in carotid vascular resistance and demonstrating the capacity of the vasculature to respond to NO (P < 0.0001, 1-way ANOVA).
Diminished response to NOS inhibition
The initial increase in blood pressure that was observed after infusion of LNNA was not sustained throughout the remaining 4 h of the experiment. Given the long half-life of LNNA (Tabrizi-Fard & Fung, 1996), this decline could not be attributed to declining concentrations of LNNA in the plasma. To confirm the effectiveness of LNNA blockade additional boluses of LNNA were given 2 h after the nitrite infusion; there were no significant further effects on arterial blood pressure or carotid flow in six fetuses. Similar results have been described previously in fetal sheep (Chlorakos et al. 1998) and it has been suggested that other physiological control mechanisms become effective that compensate for the loss of endogenous NO synthesis.
To further investigate the waning cardiovascular responses to NO blockade additional experiments were performed in which the LNNA infusion was repeated 2 days after the initial LNNA infusion. Unlike the initial responses, blood pressure, carotid flow and resistance to flow did not change significantly in response to this delayed second exposure (results from 10 fetuses, 5 from the LNNA + nitrite group and 5 from the LNNA + saline group; Fig. 6). A statistical comparison of responses in the first and late second exposures to LNNA demonstrated lesser changes in blood pressure (2-way ANOVA, P = 0.002), carotid flow (P = 0.007) and carotid resistance (P = 0.025) for the second LNNA exposure. The result is consistent with adaptations of the fetal cardio-vasculature to NOS blockade that persist for days.
Figure 6. Arterial blood pressure, carotid flow and carotid resistance in response to initial NOS blockade with intravenous LNNA infusion (squares) and a repeat exposure to intravenous LNNA 2 days later (circles).
Open symbols represent time points that were significantly different from the baseline (−135 min) value (1-way ANOVA with Bonferroni post hoc analysis). The significant increases in blood pressure, decreases in carotid flow and increases in arterial resistance seen after the first exposure to LNNA were not seen with re-exposure to LNNA 2 days later. *Significant difference between 1st exposure and 2nd exposure (2-way ANOVA with Bonferroni post hoc test).
One possible mechanism for diminished responses to NOS inhibition, previously proposed in adult animals, is increased production of and sensitivity to calcitonin gene-related peptide (CGRP), a potent vasodilator (Yallampalli et al. 1996; Gangula et al. 1997; Supowit et al. 1998). To investigate this possibility, we measured CGRP concentrations in plasma samples collected prior to the first exposure to LNNA and then again prior to the second exposure 2 days later. Plasma CGRP concentrations were significantly higher in all fetuses 2 days after the initial exposure to LNNA (P = 0.03, paired t test in six fetal sheep; Fig. 7).
Figure 7. Plasma CGRP concentrations prior to the initial infusion of LNNA (Pre-LNNA) and the second infusion of LNNA 2 days later (Post-LNNA).
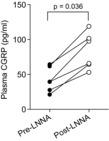
Plasma CGRP concentrations increased significantly in plasma collected 2 days after exposure to LNNA (paired t test).
Discussion
This study finds that intravascular nitrite is not a vasodilator in the cephalic vasculature of fetal sheep at concentrations ranging from physiological to nearly two orders of magnitude above the physiological range. This result, which was observed both with and without blockade of endogenous NOS activity, differs from previous experiments in the adult human forearm that demonstrated nitrite is a vasodilator at concentrations similar to those used in the current experiments.
Role of nitrite in regulation of vascular tone
While nitrite has been recognized as a vasodilator at pharmacological concentrations for at least six decades (Furchgott & Bhadrakom, 1953), more recent evidence indicates that it also reduces vascular tone at physiological concentrations (Cosby et al. 2003; Dejam et al. 2007; Mack et al. 2008); although at least one previous report using similar methodology found no vasodilatory effects of nitrite (Lauer et al. 2001). In addition, isolated arteries dilate in response to nitrite added to tissue baths at concentrations in the low micromolar range (Crawford et al. 2006; Isbell et al. 2007).
The dilating effects of nitrite involve stimulation of vascular cGMP production and are inhibited by NO scavengers, indicating that nitrite acts by conversion to NO. It has been hypothesized that this conversion occurs by reaction of nitrite with deoxyhaemoglobin in the blood (Reaction 1), constituting a haemoglobin-mediated system for the local detection of hypoxia and subsequent production of NO and local vasodilatation which would serve to match O2 delivery with O2 need (Gladwin et al. 2006). In support of this hypothesis, experiments have shown that the vasodilating effect of nitrite on isolated arteries is potentiated in the presence of deoxygenated red blood cells (Cosby et al. 2003; Crawford et al. 2006; Isbell et al. 2007; Cantu-Medellin et al. 2011).
In the current study, however, we did not observe dilatory effects of nitrite despite the supra-physiological concentrations and the fact that Reaction 1 is known to proceed ∼2-fold faster with fetal haemoglobin compared to adult haemoglobin (Blood et al. 2009). These results may indicate that the rate of Reaction 1 is not a sufficient determinant of the vasodilating effects of nitrite in the sheep fetus. There are several points consistent with this idea. First, under physiological conditions the rate at which NO would be expected to be produced by Reaction 1 (Huang et al. 2005; Blood et al. 2009) is six to seven orders of magnitude slower than the rate at which free NO either binds tightly to deoxyhaemoglobin (Cassoly & Gibson, 1975; thus limiting the vasoactivity of the NO), or reacts with oxyhaemoglobin (Eich et al. 1996) to produce nitrate, which has no vasoactivity. Thus, the rapid rates of reactions between NO and haemoglobin would preclude any production of vasoactive amounts of NO from Reaction 1. To escape this limitation there is the possibility that the reaction is compartmentalized so the NO produced would be protected from exposure to other haemoglobins until it is exported from the erythrocyte. The possibility of compartmentalization of Reaction 1 has been proposed previously (Gladwin et al. 2006; Vitturi et al. 2009; Jensen & Rohde, 2010; Salhany, 2013), and gains circumstantial credibility from reports demonstrating that deoxyhaemoglobin associates closely with the anion exchanger (Rauenbuehler et al. 1982; Walder et al. 1984) through which nitrite enters and exits the erythrocyte (Vitturi et al. 2009; Jensen & Rohde, 2010). This putative mechanism of nitrite reduction and NO export raises the possibility of developmental or interspecies differences that could account for the lack of effect of nitrite observed in the present study. For example, one might hypothesize that fetal or sheep haemoglobin/anion exchange proteins lack the necessary components needed to facilitate the export of NO produced from nitrite.
Another possible explanation for the lack of vasodilatory effects of nitrite in our experiments, in contrast with studies in the human forearm, is that the cerebral vasculature may be less sensitive to nitrite than the peripheral vasculature. However, we observed no significant change in fetal arterial blood pressure or heart rate, the primary determinant of fetal cardiac output, indicating there were no major decreases in total peripheral vascular resistance.
There is also the possibility that the reduction of nitrite to NO is not confined to blood, but may also occur within smooth muscle cells, for example, when nitrite ions have entered the cytoplasm. The haem-containing proteins myoglobin and cytoglobin are both known to act as nitrite reductases (Rassaf et al. 2007; Alzawahra et al. 2008). In addition, xanthine oxidase, aldehyde oxidase and cytochrome c can also converte nitrite to NO, and there is evidence that all of these proteins facilitate nitrite-mediated vasodilatation within the vascular smooth muscle itself (Alzawahra et al. 2008; Li et al. 2009, 2012; Ormerod et al. 2011; Totzeck et al. 2012; Liu et al. 2013). Again, possible developmental or interspecies differences in the concentrations of these proteins and in their nitrite reductase activity in fetal vascular smooth muscle do not appear to have been examined.
Effect of NOS inhibition on cephalic vascular resistance and circulating nitrite
The normal fetal cardiovascular system is in a high-flow and low-resistance state, carrying 3-to 4-fold more blood flow per kilogram of body weight at half of the arterial blood pressure of the adult. NO production from NOS plays an essential role in maintaining this low vascular resistance in the fetal sheep as early as 60% completion of gestation (Harris et al. 2001). In the near-term fetus, NOS blockade results in significantly increased constriction of the vasculature of most peripheral organs studied (Green et al. 1996; Harris et al. 2001), as well as the brain (Green et al. 1996; van Bel et al. 1997; Harris et al. 2001; Hunter et al. 2003). The present study is in agreement with these earlier reports in that NOS blockade resulted in a 2-fold increase in resistance to blood flow in the vasculature supplied by the carotid artery.
The present study shows a refractoriness of cephalic vessels in response to intra-arterial nitrite and minimal systemic responses to circulating nitrite. Even at 20-fold systemic concentrations, circulating nitrite did not induce systemic hypotension, as they would typically in most adult animals and humans, nor did they induce changes in heart rate, which suggests only minimal changes in cardiac output.
Delayed adaptive haemodynamic response to NOS blockade
Our observation that the hypertension caused by the LNNA bolus begins to subside as early as 60 min after the LNNA bolus has been reported once previously in fetal sheep (Chlorakos et al. 1998). Consistent with this previous report, we observed that the hypertensive response to NOS blockade was virtually absent upon repeat administration of LNNA 2 days after the first exposure. This finding suggests that loss of NOS activity in the fetus activates compensatory vasodilating mechanisms that effectively lower blood pressure within 1 h after NOS inhibition and persist for at least 2 days. A similar adaptive response to chronic hypertension caused by NOS blockade (Yallampalli et al. 1996; Buhimschi et al. 1997; Gangula et al. 1997; Kinsella et al. 2006) or subtotal nephrectomy (Gangula et al. 1997; Supowit et al. 1998) has been observed previously.
The compensatory responses to chronic hypertension in adult animals are mediated, at least in part, by increased sensitivity of the vascular smooth muscle to CGRP and increased production of CGRP (Gangula et al. 1997; Supowit et al. 1998). In addition, the hypertensive effects of NOS inhibition are blocked by administration of intravenous CGRP in pregnant rats (Yallampalli et al. 1996). Although the role of CGRP in compensating for chronic hypertension has not been studied in the fetus, taken together with the above studies in adult rats, the present observation of abolished hypertensive responses to NOS inhibition, along with increased plasma CGRP concentrations, provides a plausible explanation for both the fall in blood pressure during the study protocol and the lack of response to LNNA upon re-challenge.
In addition, other compensatory mechanisms following LNNA application may be effective in preventing the ‘rebound effects’ of systemic blood pressure and carotid flow that were observed in fetuses that did not receive LNNA when the nitrite infusion was stopped (Fig. 4). These haemodynamic changes appear to be cardiogenic, as the increase in blood pressure and carotid flow was associated with an increase in heart rate. The increase in carotid flow does not reflect local vasodilatation because carotid vascular resistance did not change significantly.
Limitations of the study
This study has not pinpointed the reasons why the fetal ovine circulation should be particularly unresponsive to the vasodilating properties of nitrite. One possibility is developmental, whereby fetuses are unresponsive, but would become responsive with ageing. Another possibility is a difference in species, whereby sheep are less responsive than humans and other species. Whichever might be the case, the difference is likely to reside in the properties of the vascular smooth muscle of fetal sheep. Differences in the concentration and/or activity of nitrite-reducing proteins such as myoglobin and cytoglobin may be suggested as possible candidates to explain the difference.
Conclusions
In summary, this study demonstrates that, although nitrite reacts with fetal deoxyhaemoglobin to produce NO at a rate approximately 2-fold faster than adult haemoglobin (Blood et al. 2009), nitrite is not a physiological vasodilator in the ovine cephalic circulation. This conclusion suggests that the rate of nitrite reaction with deoxyhaemoglobin is not a limiting factor in nitrite-induced vasodilatation that has been reported previously in the human forearm. Further investigation into the mechanism underlying differential responses of the fetal sheep and adult human vasculature to nitrite, especially those related to vascular smooth muscle, may lead to a deeper understanding of the mechanisms by which nitrite induces vasodilatation in the adult.
Key points
Recent evidence in adult humans demonstrates that nitrite, at physiological concentrations, can be converted into vasodilating amounts of NO, thus constituting an alternative to NO production by NO synthases.
Nitrite reacts with deoxyhaemoglobin to produce NO, a reaction proposed to mediate the vasodilating effects of nitrite. We have demonstrated previously that the rate of this reaction is ∼2-fold faster with fetal haemoglobin than adult haemoglobin. Thus, we hypothesized that nitrite would be a potent vasodilator in the cephalic vasculature served by the carotid artery in the fetal sheep.
In conflict with human adult forearm studies, we find that nitrite is not a vasodilator in the fetal sheep cephalic vasculature, despite the fact that nitrite is converted to NO more efficiently by fetal haemoglobin.
The results suggest that the vasodilatory effects of nitrite are age-and species-specific, and that the reaction of nitrite with deoxyhaemoglobin is not rate limiting with respect to its ability to decrease vascular tone.
Acknowledgments
None Declared.
Glossary
- CGRP
calcitonin gene-related peptide
- LNNA
l-nitro-arginine
- NO
nitric oxide
- NOS
nitric oxide synthase
- PROLI-NO
1-(hydroxy-NNO-azoxy)-l-proline
Additional information
Competing interests
The authors have no conflicts of interest to disclose.
Author contributions
G.T.T., H.J.S., G.G.P. and A.B.B. contributed to the conception and design of these experiments. The collection, analysis and interpretation of various data were performed by all authors. Drafting and revising of the manuscript were performed by G.T.T., H.J.S., G.G.P. and A.B.B. All authors approved the final version of the manuscript.
Funding
This research was paid for by intramural funding from the John Mace Pediatric Research Grant Fund of the Loma Linda University School of Medicine Department of Pediatrics, and from the National Heart, Lung, and Blood Institute of the NIH (R01HL095973, to A.B.B.).
References
- Alzawahra WF, Talukder MA, Liu X, Samouilov A, Zweier JL. Heme proteins mediate the conversion of nitrite to nitric oxide in the vascular wall. Am J Physiol Heart Circ Physiol. 2008;295:H499–H508. doi: 10.1152/ajpheart.00374.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AB, Power GG. In vitro and in vivo kinetic handling of nitrite in blood: effects of varying hemoglobin oxygen saturation. Am J Physiol Heart Circ Physiol. 2007;293:H1508–H1517. doi: 10.1152/ajpheart.01259.2006. [DOI] [PubMed] [Google Scholar]
- Blood AB, Tiso M, Verma ST, Lo J, Joshi MS, Azarov I, Longo LD, Gladwin MT, Kim-Shapiro DB, Power GG. Increased nitrite reductase activity of fetal versus adult ovine hemoglobin. Am J Physiol Heart Circ Physiol. 2009;296:H237–H246. doi: 10.1152/ajpheart.00601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi C, Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE. Contrasting effects of diethylenetriamine–nitric oxide, a spontaneously releasing nitric oxide donor, on pregnant rat uterine contractility in vitro versus in vivo. Am J Obst Gynecol. 1997;177:690–701. doi: 10.1016/s0002-9378(97)70166-2. [DOI] [PubMed] [Google Scholar]
- Cantu-Medellin N, Vitturi DA, Rodriguez C, Murphy S, Dorman S, Shiva S, Zhou Y, Jia Y, Palmer AF, Patel RP. Effects of T-and R-state stabilization on deoxyhemoglobin-nitrite reactions and stimulation of nitric oxide signaling. Nitric Oxide. 2011;25:59–69. doi: 10.1016/j.niox.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassoly R, Gibson Q. Conformation, co-operativity and ligand binding in human hemoglobin. J Mol Biol. 1975;91:301–313. doi: 10.1016/0022-2836(75)90382-4. [DOI] [PubMed] [Google Scholar]
- Chlorakos A, Langille BL, Adamson SL. Cardiovascular responses attenuate with repeated NO synthesis inhibition in conscious fetal sheep. Am J Physiol Heart Circ Physiol. 1998;274:H1472–H1480. doi: 10.1152/ajpheart.1998.274.5.H1472. [DOI] [PubMed] [Google Scholar]
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- Feelisch M, Rassaf T, Mnaimneh S, Singh N, Bryan NS, Jourd’Heuil D, Kelm M. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. FASEB J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Bhadrakom S. Reactions of strips of rabbit aorta to epinephrine, isopropylarterenol, sodium nitrite and other drugs. J Pharmacol Exp Ther. 1953;108:129–143. [PubMed] [Google Scholar]
- Gangula PR, Supowit SC, Wimalawansa SJ, Zhao H, Hallman DM, DiPette DJ, Yallampalli C. Calcitonin gene-related peptide is a depressor in NG-nitro-l-arginine methyl ester-induced hypertension during pregnancy. Hypertension. 1997;29:248–253. doi: 10.1161/01.hyp.29.1.248. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- Gratton R, Carmichael L, Homan J, Richardson B. Carotid arterial blood flow in the ovine fetus as a continuous measure of cerebral blood flow. J Soc Gynecol Investig. 1996;3:60–65. doi: 10.1016/1071-5576(95)00047-X. [DOI] [PubMed] [Google Scholar]
- Green LR, Bennet L, Hanson MA. The role of nitric oxide synthesis in cardiovascular responses to acute hypoxia in the late gestation sheep fetus. J Physiol. 1996;497:271–277. doi: 10.1113/jphysiol.1996.sp021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AP, Helou S, Gleason CA, Traystman RJ, Koehler RC. Fetal cerebral and peripheral circulatory responses to hypoxia after nitric oxide synthase inhibition. Am J Physiol Regul Integr Comp Physiol. 2001;281:R381–R390. doi: 10.1152/ajpregu.2001.281.2.R381. [DOI] [PubMed] [Google Scholar]
- Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Blood AB, White CR, Pearce WJ, Power GG. Role of nitric oxide in hypoxic cerebral vasodilatation in the ovine fetus. J Physiol. 2003;549:625–633. doi: 10.1113/jphysiol.2002.038034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol. 2007;293:H2565–H2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- Jensen FB, Rohde S. Comparative analysis of nitrite uptake and hemoglobin-nitrite reactions in erythrocytes: sorting out uptake mechanisms and oxygenation dependencies. Am J Physiol Regul Integr Comp Physiol. 2010;298:R972–R982. doi: 10.1152/ajpregu.00813.2009. [DOI] [PubMed] [Google Scholar]
- Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, George TN, Southgate WM, Carriedo H, Couser RJ, Mammel MC, Hall DC, Pappagallo M, Sardesai S, Strain JD, Baier M, Abman SH. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. New Eng J Med. 2006;355:354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Hemann C, Abdelghany TM, El-Mahdy MA, Zweier JL. Characterization of the mechanism and magnitude of cytoglobin-mediated nitrite reduction and nitric oxide generation under anaerobic conditions. J Biol Chem. 2012;287:36623–36633. doi: 10.1074/jbc.M112.342378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kundu TK, Zweier JL. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J Biol Chem. 2009;284:33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Tong J, Zweier JR, Follmer D, Hemann C, Ismail RS, Zweier JL. Differences in oxygen-dependent nitric oxide metabolism by cytoglobin and myoglobin account for their differing functional roles. FEBS J. 2013;280:3621–3631. doi: 10.1111/febs.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo LD, Pearce WJ. High altitude, hypoxic-induced modulation of noradrenergic-mediated responses in fetal and adult cerebral arteries. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:683–694. doi: 10.1016/s1095-6433(98)01006-x. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E. NO-synthase independent NO generation in mammals. Biochem Biophys Res Commun. 2010;396:39–45. doi: 10.1016/j.bbrc.2010.02.136. [DOI] [PubMed] [Google Scholar]
- Mack AK, McGowan VR, II, Tremonti CK, Ackah D, Barnett C, Machado RF, Gladwin MT, Kato GJ. Sodium nitrite promotes regional blood flow in patients with sickle cell disease: a phase I/II study. Br J Haematol. 2008;142:971–978. doi: 10.1111/j.1365-2141.2008.07259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod JO, Ashrafian H, Maher AR, Arif S, Steeples V, Born GV, Egginton S, Feelisch M, Watkins H, Frenneaux MP. The role of vascular myoglobin in nitrite-mediated blood vessel relaxation. Cardiovasc Res. 2011;89:560–565. doi: 10.1093/cvr/cvq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, Gladwin MT, Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med. 2006;41:541–548. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100:1749–1754. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- Rauenbuehler PB, Cordes KA, Salhany JM. Identification of the hemoglobin binding sites on the inner surface of the erythrocyte membrane. Biochim Biophys Acta. 1982;692:361–370. doi: 10.1016/0005-2736(82)90385-6. [DOI] [PubMed] [Google Scholar]
- Rothstein RW, Longo LD. Respiration in the fetal-placental unit. In: Cowett RM, editor. Principles of Perinatal–Neonatal Metabolism. 2nd edn. New York: Springer-Verlag; 1998. pp. 451–486. [Google Scholar]
- Rybakowski C, Eisermann K, Tchirikov M, Schroder HJ. Efficacy of carotid collateral perfusion in anaesthetized sheep fetuses. Placenta. 2000;21:718–725. doi: 10.1053/plac.2000.0565. [DOI] [PubMed] [Google Scholar]
- Salhany JM. The oxidative denitrosylation mechanism and nitric oxide release from human fetal and adult hemoglobin, an experimentally based model simulation study. Blood Cells Mol Dis. 2013;50:8–19. doi: 10.1016/j.bcmd.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Supowit SC, Zhao H, Hallman DM, DiPette DJ. Calcitonin gene-related peptide is a depressor in subtotal nephrectomy hypertension. Hypertension. 1998;31:391–396. doi: 10.1161/01.hyp.31.1.391. [DOI] [PubMed] [Google Scholar]
- Tabrizi-Fard MA, Fung HL. Pharmacokinetics and steady-state tissue distribution of L-and D-isomers of nitroarginine in rats. Drug Metab Dispos. 1996;24:1241–1246. [PubMed] [Google Scholar]
- Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bel van F, Sola A, Roman C, Rudolph AM. Perinatal regulation of the cerebral circulation: role of nitric oxide and prostaglandins. Pediatr Res. 1997;42:299–304. doi: 10.1203/00006450-199709000-00009. [DOI] [PubMed] [Google Scholar]
- Vitturi DA, Teng X, Toledo JC, Matalon S, Lancaster JR, Jr, Patel RP. Regulation of nitrite transport in red blood cells by hemoglobin oxygen fractional saturation. Am J Physiol Heart Circ Physiol. 2009;296:H1398–H1407. doi: 10.1152/ajpheart.01303.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder JA, Chatterjee R, Steck TL, Low PS, Musso GF, Kaiser ET, Rogers PH, Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984;259:10238–10246. [PubMed] [Google Scholar]
- Wang X, Bryan NS, MacArthur PH, Rodriguez J, Gladwin MT, Feelisch M. Measurement of nitric oxide levels in the red cell: validation of tri-iodide-based chemiluminescence with acid-sulfanilamide pretreatment. J Biol Chem. 2006;281:26994–27002. doi: 10.1074/jbc.M603953200. [DOI] [PubMed] [Google Scholar]
- Yallampalli C, Dong YL, Wimalawansa SJ. Calcitonin gene-related peptide reverses the hypertension and significantly decreases the fetal mortality in pre-eclampsia rats induced by NG-nitro-L-arginine methyl ester. Hum Reprod. 1996;11:895–899. [PubMed] [Google Scholar]


