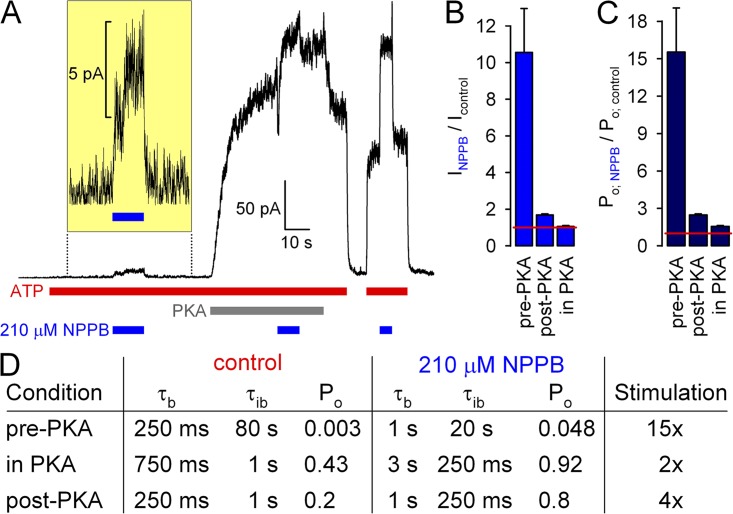
Figure 8.
Phosphorylation dependence of NPPB stimulation of WT CFTR reflects nonlinear dependence of Po on opening and closing rates. (A) Macroscopic WT CFTR currents in 2 mM ATP at +60 mV, and applications of 210 µM NPPB before exposure to (current scale magnified in inset), in the presence of, and after removal of 300 nM PKA catalytic subunit. (B and C) Fractional effects of 210 µM NPPB on (B) steady-state macroscopic currents and (C) Po of unphosphorylated (pre-PKA), fully phosphorylated (in PKA), and partially dephosphorylated (post-PKA) WT CFTR channels at +60 mV. (D) Table summarizing expected changes in gating parameters assuming a constant (phosphorylation-independent) fourfold increase in τb and fourfold decrease in τib in the presence of 210 µM NPPB, as measured in this study for the post-PKA condition at −120 mV (Fig. 3; slightly smaller, approximately threefold, effects were measured at +60 mV; Fig. 6). Control parameters (left column) reflect typical values measured for WT CFTR in this and previous studies (e.g., Csanády et al., 2005; Szollosi et al., 2010); the post-PKA values in NPPB (right column, bottom row) reflect the values measured in Fig. 3.