Figure 1. Domain organization of sGC subunits and truncations.
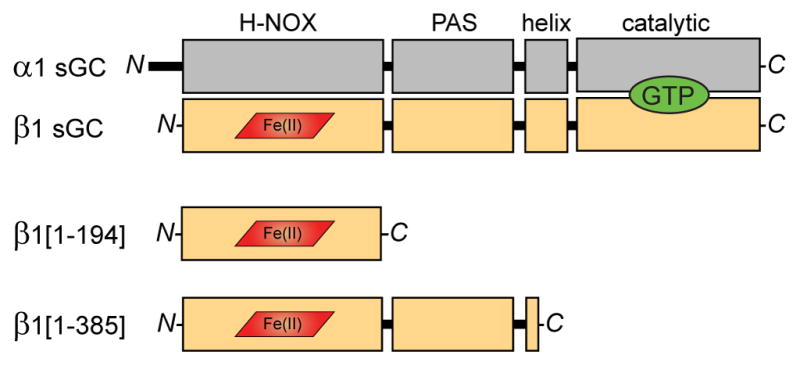
The sGC holoenzyme consists of two homologous subunits, α1 (gray) and β1 (tan), each composed of four domains. NO binds to the ferrous heme cofactor (red) of the β1 H-NOX domain. Cyclization of GTP (green) to cGMP occurs in the C-terminal catalytic domains. Truncations of the β1 sGC subunit preserve NO-binding. β1[1-194] comprises the minimal heme-binding H-NOX domain. β1[1-385] includes the associated PAS domain and a portion of the helical domain.
