Figure 5. Absence of endothelial LPP3 promotes vascular leak.
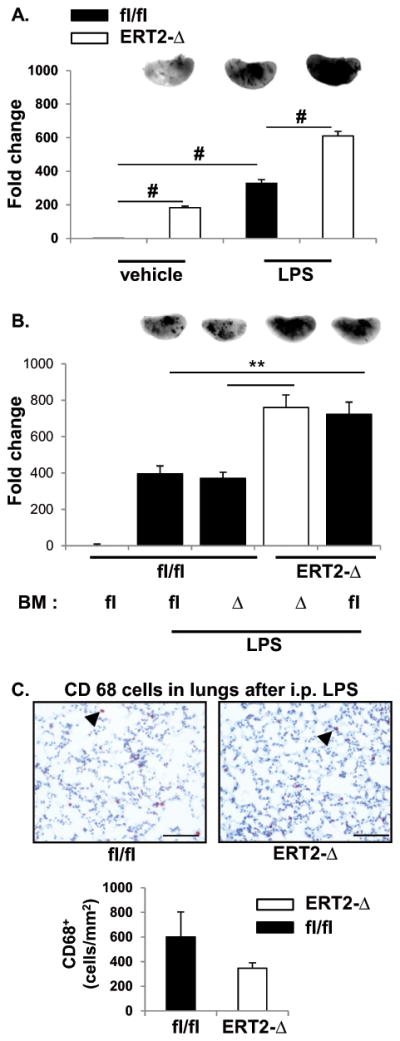
(A) LPS-induced lung endothelial permeability and accumulation of extravascular protein was measured by accumulation of EBD in lungs 2 hours after vehicle or LPS administration to Ppap2bfl/fl (fl/fl) or ERT2-Ppap2bΔ (ERT2-Δ) mice. The amount of EBD in left lung was measured by near-infrared imaging (LiCor) and is reported as mean ± SD. Representative images for each treatment and genotype are included at the top of the bar graphs. More EBD accumulates in the lung of ERT2-Δ mice at baseline (vehicle) and after LPS challenge. # P<0.05 by ANOVA. (B) Chimeric mice were created by bone marrow (BM) transplantation between fl/fl or ERT2-Δ animals, and the mice then treated with tamoxifen. Protein leak after vehicle or LPS treatment was measured as described above. The permeability defect segregated with absence of LPP3 in recipient mice (i.e., blood vessels) and not with the genotype of BM cells. # P<0.05 by ANOVA. (C) CD68-positive macrophage staining (arrowhead) in lung tissue 6 hours after LPS. The number of macrophages was scored per mm2 and graphed as mean ± SD. At the dose of LPS administered (2 mg/kg), the permeability defect in endothelial cells did not result in accumulation of macrophages in lung tissue as visualized by CD68 staining.
