Figure 1.
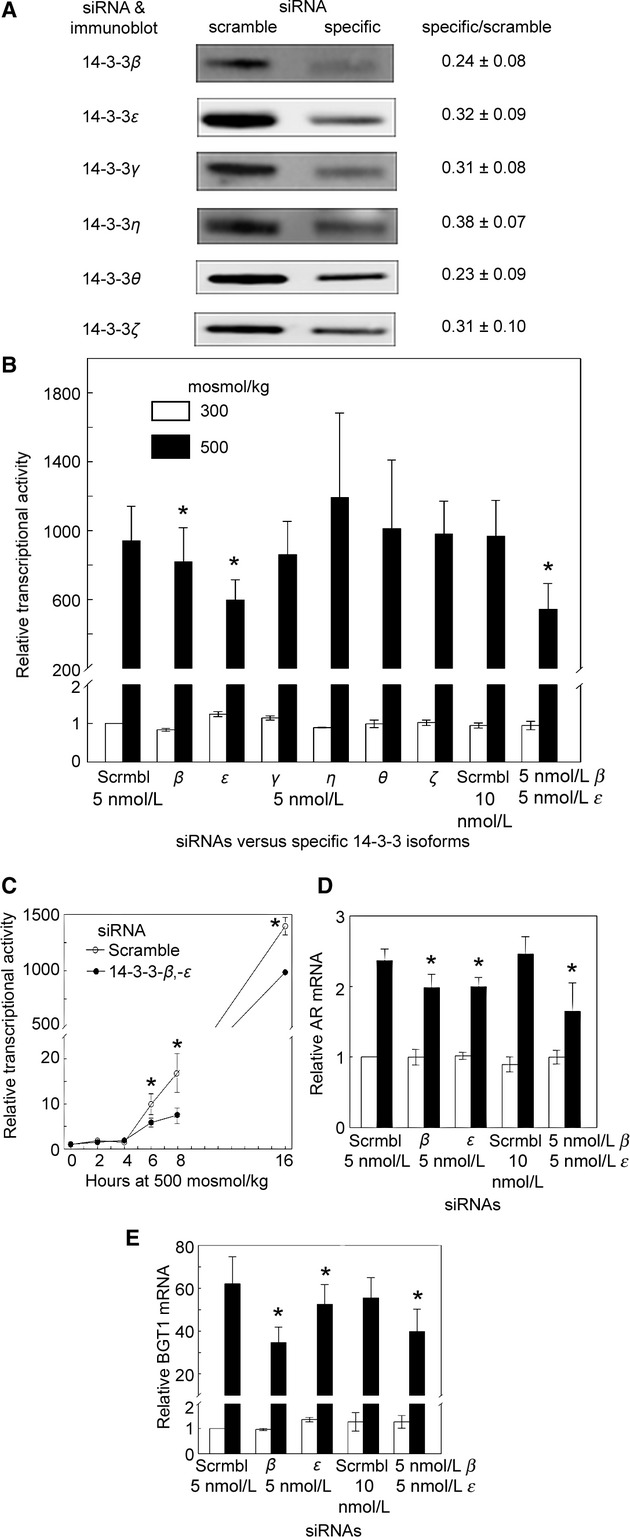
(A) Effectiveness of siRNAs versus 14‐3‐3s. HEK293 cells were transfected with 5 nmol/L siRNAs for 3 days, then cell proteins were extracted, and abundance of 14‐3‐3 proteins measured by Western analysis. siRNAs knock 14‐3‐3s down by 62–77%. Center panel shows representative Western blots. Right panel shows mean ± SEM, n = 3. (B) Effect of knock down of 14‐3‐3s on transcriptional activity of NFAT5. HEK293 cells that stably express the ORE‐X luciferase reporter of NFAT5 transcriptional activity were used. The siRNAs were transfected for 3 days into cells incubated at 300 mosmol/kg, then the medium was changed, keeping the osmolality at 300 or increasing it to 500 mosmol/kg for 16 h by adding NaCl. None of the siRNAs significantly affect the transcriptional activity at 300 mosmol/kg, but at 500 mosmol/kg siRNAs against 14‐3‐3‐β or ‐ε inhibit NFAT5 transcriptional activity by 15% and 35%, respectively, and knock down of ‐β and ‐ε in combination inhibits ORE‐X activity by 45% (mean ± SEM, *P < 0.05, n = 3–6). (C) Time course of the high NaCl‐induced increase in NFAT5 transcriptional activity. As in (B), except that NFAT5 transcriptional activity was measured at the times shown. Effect of knock down of 14‐3‐3‐β and ‐ε on mRNA expression of the NFAT5 target genes aldose reductase (AR) (D) and BGT1 (E). As in (B), except that AR or BGT1 mRNA was measured in HEK293 cells (mean ± SEM, *P < 0.05, n = 3).
