Abstract
Background
Implantable cardioverter-defibrillator (ICD) generator replacement at the end of expected battery life accounts for a substantial proportion of all ICD implant procedures. However, little is known about predictors of mortality following ICD generator replacement.
Objective
To identify clinical and procedural factors associated with death following ICD generator replacement.
Methods
Patients from the National Cardiovascular Data Registry (NCDR®) ICD Registry™ receiving ICD generator replacements at the end of device battery life between January 1, 2005 and March 30, 2010 were eligible. Predictors of mortality were determined using multivariable Cox regression.
Results
Analysis of 111,826 patients (mean age 70.7 +12.4, 75.5% male) revealed 1-, 3-, and 5-year mortality of 9.8%, 27.0%, and 41.2%, respectively. After adjustment, atrial fibrillation (hazard ratio (HR) 1.23, 95% confidence interval (CI) 1.20 – 1.27) and congestive heart failure (HR 1.21, 95% CI 1.16 – 1.27) predicted worse survival. In addition to older age (HR 1.43 95% CI 1.41 – 1.45), several non-cardiac conditions were also associated with poorer survival, including: chronic lung disease (HR 1.53, 95% CI 1.49 – 1.57), cerebrovascular disease (HR 1.28, 95% CI 1.24 – 1.32), diabetes (HR 1.27, 95% CI 1.23 – 1.30), and lower glomerular filtration rate (HR 1.15 for each 10 unit (U) increment decline, 95% CI 1.14 – 1.16). In the absence of a non-ICD control group, risk reduction provided by ICD therapy in this cohort is not known.
Conclusions
In addition to age, atrial fibrillation, and congestive heart failure, non-cardiac comorbidities are associated with higher mortality following ICD replacement, which should be considered in the decision to undergo this procedure
Keywords: Implantable cardioverter-defibrillators, outcomes research
Introduction
Implantable cardioverter-defibrillator (ICD) implantation continues to attract intense scrutiny from clinicians, policy-makers, and the public.1 Recent publication of appropriate use criteria (AUC) for ICD implantation further argues for refined decision-making for potential recipients of new devices.2 The AUC draw in part from models derived from clinical trials,3, 4 registries,5 and academic centers6, 7 describing survival following new ICD implantation, with the hope of identifying patients at particularly high risk for death despite the presence of an ICD. However, reports from nationwide data suggest that nearly 40% of all ICD implantations are replacements of existing devices.8 Far less is known about the outcomes of these patients who are on average 3 years older than patients receiving new ICDs and have more arrhythmic comorbidities such as atrial fibrillation and ventricular tachycardia.9 More than 20% of these patients will have received shocks from their ICD prior to replacement, with uncertain implications for long-term survival.8
Despite these important clinical complexities, survival following ICD generator replacement has only recently been evaluated in detail.9 Prior analysis from the National Cardiovascular Data Registry – ICD Registry identified patients following generator replacement at the end of expected battery life to be at high risk for death compared to patients receiving new ICDs.9 This hazard persisted with and without adjustment for key clinical variables, but the contribution of comorbidities and other factors to mortality in this group remain poorly understood.
Construction of prospective studies evaluating the management of patients eligible for ICD replacement will depend in part on a clearer picture of what contributes to post-replacement mortality.10 Thus, the goals of this study were to identify clinical factors associated with mortality among patients following ICD generator replacement.
Methods
Data Source
This study analyzed data from the National Cardiovascular Data Registry (NCDR®) ICD Registry™, the details of which have been previously published.11–13 In brief, the ICD Registry™ was created in 2005 after the Centers for Medicare and Medicaid Services (CMS) national coverage decision for primary prevention ICD implantation, though most participating centers contribute data on all of their implants using data collected at the time of the procedure.. Over 850,000 procedures have been captured as of December 2011, with over 12,000 new procedures entered each month.8 All data entry for the current study was performed using the ICD Registry™ Data Collection From v1.08.14. Participating sites receive formal training on data collection and entry by the NCDR®. After submission, data are evaluated for quality and returned to sites if incomplete. Data from the ICD Registry™ have been used to address key clinical research questions in prior studies12, 15 including analysis of ICD generator replacement.9
Patient files are linked to the Social Security Death Index to determine patient vital status, which was available through 10/1/2011.
Study Population
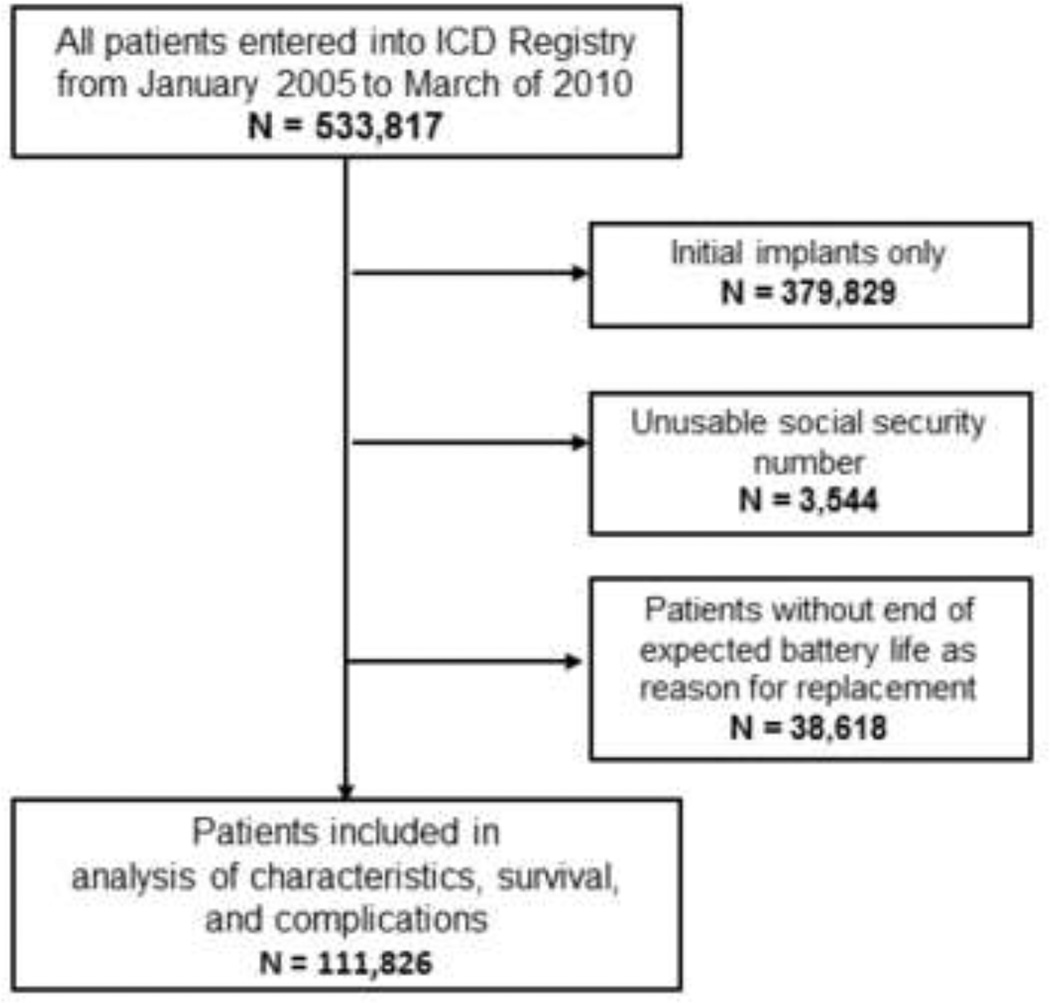
All patients receiving replacement ICDs between January 1, 2005 and March 30, 2010 were eligible for inclusion (Figure 1). Patients missing social security numbers were excluded as their vital status could not be determined. As the primary focus of this study was survival following routine replacement, subjects who did not have “end of expected battery life” as one of the reasons for replacement were also excluded. For patients with multiple entries (for initial and one or more replacement procedures) analysis of characteristics and survival focused on the first replacement procedure for which “end of expected battery life” pertained.
Figure 1.
All patients entered into the ICD Registry from January 2005 – March 2010 were eligible. Those who only received initial ICD implants and those without usable social security numbers were excluded. For those patients receiving replacement ICDs, those whose reason(s) for replacement did not include end of expected battery life were also excluded.
Variables
The ICD Registry™ collects over 130 standardized data elements describing demographic, clinical information and procedural information for each patient undergoing an ICD procedure. For this report, variables were selected a priori from the ICD Registry™ that were felt to be necessary to describe and examine the characteristics and outcomes of patients receiving replacement ICDs based on American College of Cardiology / American Heart Association guidelines for device based therapy16 as well as published literature regarding ICD outcomes (Table 1).3–6
Table 1.
Characteristics of Patients Receiving Replacement ICDs.
| Characteristic | Total n = 111,826 |
|---|---|
| Demographics | |
| Age (years) | 70.7 ± 12.4 |
| Male | 84439 (75.5%) |
| Caucasian | 97842 (87.5%) |
| Hispanic | 4112 (3.7%) |
| Clinical History | |
| Any Ischemic Heart Disease | 75609 (67.6%) |
| Prior myocardial infarction | 62015 (55.5%) |
| Previous CABG | 44439 (39.8%) |
| Prior percutaneous coronary intervention | 35006 (31.3%) |
| Congestive Heart Failure | 82474 (73.8%) |
| Non-Ischemic Dilated Cardiomyopathy | |
| No | 81066 (72.5%) |
| Yes Within the past 3 months | 1025 (0.9%) |
| Yes 3 to 9 months | 717 (0.6%) |
| Yes Greater than 9 months | 28954 (25.9%) |
| NYHA Class | |
| Class I | 22743 (20.4%) |
| Class II | 46237 (41.4%) |
| Class III | 40158 (36.0%) |
| Class IV | 2481 (2.2%) |
| Atrial Fibrillation | 47162 (42.2%) |
| Ventricular Tachycardia | |
| None | 47052 (42.1%) |
| Yes VT, Non Sustained | 32600 (29.2%) |
| Yes Monomorphic Sustained VT | 26895 (24.1%) |
| Yes Polymorphic Sustained VT | 5175 (4.6%) |
| Abnormal Sinus Node Function | 35845 (32.1%) |
| Cerebrovascular Disease | 17849 (16.0%) |
| Chronic Lung Disease | 24089 (21.5%) |
| Diabetes | 38430 (34.4%) |
| Hypertension | 81953 (73.3%) |
| Renal Failure-Dialysis | 3101 (2.8%) |
| Left ventricular ejection fraction % | 32.4 ± 13.7 |
| QRS Duration (ms) | 138.3 ± 37.3 |
| Atrioventricular Conduction | |
| Normal | 32924 (29.4%) |
| LBBB | 16149 (14.4%) |
| RBBB | 6307 (5.6%) |
| PACED | 47620 (42.6%) |
| OTHER | 8826 (7.9%) |
| Serum creatinine (mg/dL) | 1.365 ± 0.906 |
| Glomerular filtration rate (ml/min) | 63.7 ± 30.3 |
| ICD Procedure | |
| Reason for Admission | |
| Admitted for this Procedure | 103494 (92.6%) |
| Cardiac CHF | 2442 (2.2%) |
| Cardiac Other | 4570 (4.1%) |
| Noncardiac | 1212 (1.1%) |
| ICD Indication | |
| Primary Prevention | 73394 (65.6%) |
| Secondary Prevention | 38432 (34.4%) |
| ICD Type | |
| Single Chamber | 18423 (16.5%) |
| Dual Chamber | 43547 (39.0%) |
| Biventricular | 49694 (44.5%) |
| Discharge Medications | |
| ACE-Inhibitor | 58949 (53.9%) |
| Amiodarone | 20227 (18.5%) |
| ARB | 20065 (18.3%) |
| Aspirin | 67293 (61.5%) |
| Beta Blocker | 92381 (84.4%) |
| Coumadin | 39498 (36.1%) |
| Digoxin | 36410 (33.3%) |
| Diuretic | 70653 (64.6%) |
Demographic variables included age, gender, race (white vs. other), and Hispanic ethnicity. Clinical information included data from clinical history and diagnostic studies. History of the following cardiac conditions was collected: any ischemic heart disease, myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, congestive heart failure, non-ischemic dilated cardiomyopathy (never, past 3 months, past 3–9 months, over 9 months), atrial fibrillation, ventricular tachycardia (none, nonsustained, monomorphic sustained, and polymorphic sustained), and abnormal sinus node function. Functional status was rated using the New York Heart Association levels I-IV. Finally, the presence of the following comorbid conditions were ascertained: cerebrovascular disease, chronic lung disease, diabetes, hypertension, and renal failure or dialysis.
The most recent information for the following were included; the left ventricular ejection fraction (%), QRS duration (ms), atrioventricular conduction pattern (normal, left bundle branch block (LBBB), right bundle branch block (LBBB), paced, or other), serum creatinine (mg/dl), and glomerular filtration rate (GFR) (ml/min) calculated using the Modification of Diet in Renal Disease Study Group (MDRD) Study equation.17
Procedure characteristics captured in the ICD registry included whether or not the procedure also involved an upgrade to a dual-chamber or biventricular system. The reason for hospitalization during which the device was placed was categorized as follows: ICD placement, congestive heart failure (CHF), cardiac but not for CHF, and non-cardiac. Type of device (single chamber, dual chamber, or biventricular) and whether the device was for primary or secondary prevention were also ascertained. In the ICD Registry, primary prevention indicates that the patient is at risk for but has not yet had an episode of sustained ventricular tachycardia, ventricular fibrillation, or resuscitated cardiac arrest. Thus, at the time of ICD replacement, a patient whose device was originally placed for primary prevention but subsequently experienced any of these events was coded as secondary prevention. Missing data were present <0.3% of the time for all data elements.
Statistical Analysis
All baseline demographic data, clinical information, and procedural variables were described using frequencies for categorical variables and means/medians with SDs/interquartile ranges for continuous variables. Survival for the overall cohort was evaluated with the Kaplan-Meier method. For subjects who died, survival time was defined from the date of the ICD to replacement until the date of death. Subjects who did not die during the study observation period (ending 10/1/2011) were censored.
Predictors of survival were evaluated using Cox regression analysis. First, unadjusted hazard ratios and 95% confidence intervals were calculated for the following candidate variables: age (10 year increments); male gender; congestive heart failure (yes/no), NHYA heart failure class (using Class I as reference), atrial fibrillation, ventricular tachycardia (any), prior myocardial infarction, prior CABG, cerebrovascular disease, chronic lung disease, diabetes mellitus, hypertension, left ventricular ejection fraction (10 unit increments), QRS duration (5ms increments), GFR (10 unit increments), primary versus secondary prevention, and ICD type (single-chamber as reference). Next, a saturated model was created including all candidate variables, yielding adjusted hazard ratios and 95% confidence intervals. Complete case analysis was used to develop the model given the very low rate of missing variables.
Results
Baseline Patient Characteristics
From an initial pool of 533,817 procedures entered into the ICD Registry during the study period, 111,826 unique patients who received ICD replacements were eligible for analysis (see Figure 1). Only 3544 were excluded for lack of a usable social security number. The median follow-up time was 2.0 years (25–75% IQR 1.4–3.0), and the median time from initial ICD implantation was 4.6 years (25–75% IQR 3.6 – 5.8).
Table 1 presents the demographic, clinical and procedural characteristics of the study cohort at the time of their ICD replacement, which had a mean age of 70.7 +12.4 and was predominantly male (75.5%) and white (87.5%). In terms of the clinical substrate for ICD therapy, 67.6% of patients had coronary artery disease including 39.8% with a history of CABG, 31.3% prior PCI, and 55.5% prior MI. Congestive heart failure was present in 73.8% of patients, predominantly NYHA Class II (41.4%) or III (36.0%). Clinical arrhythmias were common including atrial fibrillation, any ventricular tachycardia, and sinus node dysfunction, as were diabetes and hypertension.
With regard to diagnostic studies at the time of ICD replacement, the mean left ventricular ejection fraction was 32.4 + 13.7%, mean serum creatinine was 1.3 + 0.9 mg/dL, and mean GFR was 63.7 ± 30.3 ml/min. Among patients without a paced rhythm (64,206, 57%), the median QRS was 120ms (25–75% IQR, 100–152).
Procedural Characteristics
The vast majority of patients (92.6%) presented for their ICD replacement procedure specifically, and 65.6% received their devices for primary prevention of sudden cardiac death. For 6970 (6.2%) of all patients receiving replacement ICDs, an upgrade/lead addition was also performed. Device malfunction (1422, 1.3%), Recalls (1220, 1.1%), and infections (117, 0.1%) were uncommonly identified as additional reasons for replacement.
Survival
One-, three-, and five-year mortality were 9.8%, 27.0%, and 41.2%, respectively (see Figure 2). Unadjusted and adjusted associations with mortality are detailed in Table 2. After multivariate adjustment, the cardiac conditions significantly associated with worse mortality included atrial fibrillation (HR 1.23, 95% CI 1.20 – 1.27) and the presence of congestive heart failure (HR 1.21, 95% CI 1.16 – 1.27), with NYHA Class III (HR 1.46, 95% CI 1.39 – 1.53) and Class IV (HR 2.24 95% CI 2.08--2.42) compared to Class I further worsening prognosis.
Figure 2.
Unadjusted KM curve for survival for patients receiving replacement ICDs, with the number at risk at yearly intervals of follow-up provided.
Table 2.
Unadjusted and adjusted predictors of death among patients following ICD replacement. NYHA = New York Heart Association; MI = myocardial infarction; CABG = coronary artery bypass grafting; PCI = percutaneous coronary intervention; LVEF = left ventricular ejection fraction; GFR = glomerular filtration rate.
| Unadjusted Associations | “Saturated” Adjusted Associations |
|||
|---|---|---|---|---|
| HR (CI) | p-value | HR (CI) | p-value | |
| Age (10 Yrs) | 1.583(1.564, 1.602) | <.0001 | 1.429(1.408, 1.449) | <.0001 |
| Male Gender | 1.258(1.221, 1.296) | <.0001 | 1.176(1.137, 1.217) | <.0001 |
| Congestive heart failure | 2.237(2.164, 2.313) | <.0001 | 1.213(1.161, 1.267) | <.0001 |
| NYHA Class1 Class2 Class3 Class4 |
Reference 1.638 (1.573, 1.706) 2.725 (2.620, 2.834) 5.130 (4.805, 5.477) |
<.0001 | Reference 1.125 (1.072, 1.18) 1.457 (1.386, 1.532) 2.244 (2.081, 2.42) |
<.0001 |
| Atrial fibrillation | 1.685(1.645, 1.726) | <.0001 | 1.232(1.200, 1.266) | <.0001 |
| Ventricular tachycardia | 0.988(0.964, 1.013) | 0.3512 | 1.073(1.043, 1.105) | <.0001 |
| Prior MI | 1.317(1.284, 1.35) | <.0001 | 0.986(0.957, 1.016) | 0.3700 |
| Prior CABG | 1.503(1.467, 1.54) | <.0001 | 1.076(1.046, 1.107) | <.0001 |
| Prior PCI | 1.035(1.009, 1.062) | 0.0093 | 0.938(0.911, 0.966) | <.0001 |
| Cerebrovascular disease | 1.603(1.557, 1.651) | <.0001 | 1.276(1.236, 1.318) | <.0001 |
| Chronic lung disease | 1.798(1.751, 1.846) | <.0001 | 1.529(1.486, 1.573) | <.0001 |
| Diabetes mellitus | 1.46(1.425, 1.497) | <.0001 | 1.269(1.234, 1.304) | <.0001 |
| Hypertension | 1.227(1.193, 1.262) | <.0001 | 0.986(0.956, 1.018) | 0.3953 |
| LVEF (decreasing 10 units) | 0.746(0.738, 0.755) | <.0001 | 1.186 (1.172,1.202) | <.0001 |
| QRS Duration (increasing 5 Units) | 1.037(1.036, 1.039) | <.0001 | 1.007(1.005, 1.008) | <.0001 |
| GFR (decreasing 10 Units) | 0.795(0.79, 0.799) | <.0001 | 1.149 (1.141, 1.157 | <.0001 |
| Primary Prevention | 1.138(1.109, 1.167) | <.0001 | 0.945(0.917, 0.974) | 0.0002 |
| ICD Type Single Dual Biv |
Reference 1.024 (.986, 1.064) 1.639 (1.580, 1.700) |
<.0001 | Reference .864 (.828, .902) .798 (.763, .835) |
<.0001 |
In addition to age (HR 1.43 95% CI 1.41 – 1.45), several non-cardiac comorbidities also were associated with worse mortality after multivariate adjustment. These included chronic lung disease (HR 1.53, 95% CI 1.49 – 1.57), cerebrovascular disease (HR 1.28, 95% CI 1.24 – 1.32), diabetes (HR 1.27, 95% CI 1.23 – 1.30), and renal function (HR 1.15 for each 10U decline in GFR, 95% CI 1.14 – 1.16).
With regard to device type, both dual-chamber and biventricular devices were associated with worse survival compared with single-chamber devices prior to adjustment, but after adjustment both were protective (dual chamber HR 0.86, 95% CI 0.83–0.90; biventricular HR 0.80, 95% CI 0.76 – 0.84).
Discussion
This study reports, for the first time, the demographic, clinical, and procedural variables associated with survival in in a nationwide sample of patients following routine replacement of ICD generators at the end of expected battery life. We found that atrial fibrillation, heart failure, and left ventricular ejection fraction were independently associated with poorer survival in this cohort. In addition, non-cardiac comorbidities including chronic lung disease, cerebrovascular disease, diabetes, and worse renal function were also independently associated with worse survival. Overall, over 40% of patients died within 5 years. These findings underscore the importance of evaluating patients’ entire clinical history at the time of ICD generator replacement, with particular attention to accumulated comorbidities that may limit life expectancy and the potential of benefiting from ongoing treatment with an ICD.10
These results build upon prior attempts to characterize survival among recipients of ICDs, though nearly all of these studies excluded patients receiving replacement ICDs.1 In agreement with our data, models derived from institutional cohorts6, 7 identified cardiovascular comorbidities such as atrial fibrillation and heart failure severity as well as age and renal function as predictive of mortality after initial ICD implant, though not COPD or diabetes. Chronic lung disease and diabetes did emerge from the largest study of new ICD implants, however. Bilchik et al assessed survival among 45,884 ICD recipients using Medicare data to developed their SHOCKED algorithm incorporating age >75 years, NYHA Class III, AF, chronic kidney disease, left ventricular ejection fraction <20%, COPD and diabetes.5 In this model, COPD (HR1.70; 95% CI 1.61 –1.80) and diabetes (HR 1.43; 95% CI 1.36 – 1.50) contributed to mortality out to 4 years. Similarly, Lee et al evaluated survival among 2467 new ICD implants in a Canadian cohort. In addition to age, heart failure, peripheral arterial disease, and renal disease, Lee et al found non-cardiac comorbidities including chronic lung disease, cancer, rheumatologic disease, and microvascular complications from diabetes (but not diabetes alone) retained predictive power after adjustment.18 In a separate study drawing upon older data (devices implanted 2001–2004), Stein et al found diabetes to remain predictive in a multivariate model evaluating 1-year survival (HR 1.68, 95% CI 1.18–2.18, P=0.0004).19
Critically, accumulated comorbidities among ICD recipients influence not only survival but also benefit from the device in randomized trial populations. For example, short- and long-term evaluation of the MADIT-II study demonstrated that the subgroup with the highest number of clinical predictors (from a model including age, renal function, atrial fibrillation, heart failure class, and QRS width) had no survival advantage with ICD therapy.4, 20 Levy et al found a similar pattern among patients from the SCD-HeFT study in which patients were grouped by risk using the Seattle Heart Failure Model.21 Again, no benefit was seen in the highest risk group. These models, however, did not include many of the non-cardiac conditions noted in observational studies of new implants – and now confirmed in our evaluation of ICD replacement. In addition, the MADIT-II and SCD-HeFT models evaluated only single-chamber systems, whereas our data include dual- and biventricular devices. Our finding of an excess hazard for each of these device types before adjustment that become protective after adjustment suggests that selection of both systems depends in part on comorbidities and may influence outcome substantially. Thus, prospective studies addressing both clinical and cost-effectiveness of ICD replacement should include variables including chronic lung disease, cerebrovascular disease, and diabetes as well as single-, dual-, and biventricular devices
Our study should be interpreted in context with potential limitations. Though this cohort was largely white and male, the ICD Registry™ represents a nationwide sample of ICD recipients in the United States, and thus reflects current implantation patterns. Our cohort included patients who necessarily survived to the point of ICD replacement, and thus description of their comorbidities and disease severity is conditional on their remaining healthy enough to arrive at this clinical decision. As battery life for generator models improves, the influence of this greater time to replacement on comorbidities may be uncertain. We did not have a control group of patients without ICDs, and could not identify cause of death (e.g. arrhythmic versus nonarrhythmic) from SSDI alone. Thus, we are unable to describe survival in our population in the absence of device-based therapy. Accordingly, our findings alone should not guide clinical decision-making for patients eligible for ICD replacement. However, these data may support discussions with potential ICD recipients about life expectancy and accumulated comorbidity, and will help inform prospective studies designed to refine clinical indications and outcomes following ICD replacement.
Conclusion
Survival following ICD replacement is influenced by atrial fibrillation and heart failure severity, and also non-cardiac comorbidities including chronic lung disease, cerebrovascular disease, diabetes, and renal function.
Acknowledgments
Funding Sources: Dr. Kramer is the Lois Green Scholar at the Hebrew SeniorLife Institute for Aging Research, and is additionally supported by an award from the John A. Hartford Foundation and a career development award from the Harvard Clinical and Translational Science Center. Dr. Mitchell is supported by NIH-NIA K24AG033640.
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com. ICD Registry™ is an initiative of the American College of Cardiology Foundation and the Heart Rhythm Society.
The authors report the following additional disclosures:
DBK, KFK, PAN, SLN, PJZ, SLM: none
JAS: Reports a contract from the American College of Cardiology Foundation to provide analytic support for the National Cardiovascular Data Registry.
AEB: St. Jude Medical - honoraria for serving on DSMB for a clinical trial (unrelated to ICDs); Medtronic - honoraria for speaking at educational symposia and serving on Events committee for a clinical trial (not related to ICDs); Boston Scientific - honoraria for serving on Events Committee for a clinical trial (not related to ICDs); MEJ: Medtronic – honoraria for educational courses and small advisory input;Biotronik honoraria for educational courses
MRR: Medtronic – research support and consulting income
References
- 1.Hohnloser SH, Israel CW. Current evidence base for use of the implantable cardioverterdefibrillator. Circulation. 2013 Jul 9;128:172–183. doi: 10.1161/CIRCULATIONAHA.112.000547. [DOI] [PubMed] [Google Scholar]
- 2.Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013 Mar 26;61:1318–1368. doi: 10.1016/j.jacc.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007 Sep 18;50:1150–1157. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008 Jan 22;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 5.Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012 Oct 23;60:1647–1655. doi: 10.1016/j.jacc.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer DB, Friedman PA, Kallinen LM, et al. Development and validation of a risk score to predict early mortality in recipients of implantable cardioverter-defibrillators. Heart Rhythm. 2012 Sep 3;9:42–46. doi: 10.1016/j.hrthm.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Parkash R, Stevenson WG, Epstein LM, Maisel WH. Predicting early mortality after implantable defibrillator implantation: a clinical risk score for optimal patient selection. Am Heart J. 2006 Feb;151:397–403. doi: 10.1016/j.ahj.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Kremers MS, Hammill SC, Berul CI, et al. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013 Apr;10:e59–e65. doi: 10.1016/j.hrthm.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 9.Kramer DB, Kennedy KF, Noseworthy PA, et al. Characteristics and Outcomes of Patients Receiving New and Replacement Implantable Cardioverter-Defibrillators: Results From the NCDR. Circ Cardiovasc Qual Outcomes. 2013 Jul;6:488–497. doi: 10.1161/CIRCOUTCOMES.111.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer DB, Buxton AE, Zimetbaum PJ. Time for a change--a new approach to ICD replacement. N Engl J Med. 2012 Jan 26;366:291–293. doi: 10.1056/NEJMp1111467. [DOI] [PubMed] [Google Scholar]
- 11.Hammill SC, Kremers MS, Stevenson LW, et al. Review of the Registry's Fourth Year, Incorporating Lead Data and Pediatric ICD Procedures, and Use as a National Performance Measure. Heart Rhythm. 2010 Jul 18; doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Al-Khatib SM, Hellkamp A, Curtis J, et al. Non-evidence-based ICD implantations in the United States. JAMA. 2011 Jan 5;305:43–49. doi: 10.1001/jama.2010.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Khatib SM, Hellkamp A, Bardy GH, et al. Survival of patients receiving a primary prevention implantable cardioverter-defibrillator in clinical practice vs clinical trials. JAMA. 2013 Jan 2;309:55–62. doi: 10.1001/jama.2012.157182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cardiovascular Data Registry - ICD Registry website. [Accessed July 31, 2012]; Available at https://www.ncdr.com/WebNCDR/ICD/default_ssl.aspx.
- 15.Fein AS, Wang Y, Curtis JP, Masoudi FA, Varosy PD, Reynolds MR. Prevalence and predictors of off-label use of cardiac resynchronization therapy in patients enrolled in the National Cardiovascular Data Registry Implantable Cardiac-Defibrillator Registry. J Am Coll Cardiol. 2010 Aug 31;56:766–773. doi: 10.1016/j.jacc.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008 May 27;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Lee DS, Tu JV, Austin PC, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007 Jun 26;49:2408–2415. doi: 10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 19.Stein KM, Mittal S, Gilliam FR, et al. Predictors of early mortality in implantable cardioverter-defibrillator recipients. Europace. 2009 Jun;11:734–740. doi: 10.1093/europace/eup055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol. 2012 Jun 5;59:2075–2079. doi: 10.1016/j.jacc.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 21.Levy WC, Lee KL, Hellkamp AS, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009 Sep 8;120:835–842. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]