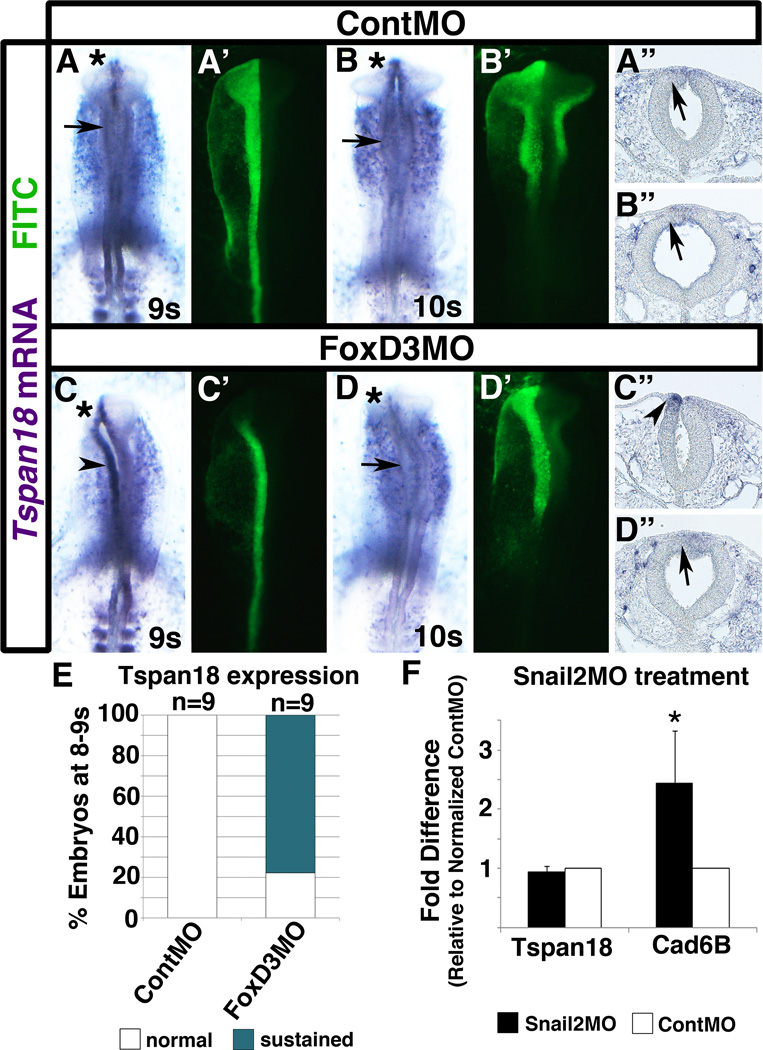
Fig. 2. Tspan18 downregulation is initially delayed in the absence of FoxD3.
Whole mount images (A-D, A’-D’) and transverse sections (A”-D”) of embryos unilaterally electroporated with ContMO (A,B) or FoxD3MO (C,D) at late gastrula and processed by in situ hybridization for Tspan18 after harvest. At 9 somites (s; A) and 10s (B), Tpsan18 mRNA is absent (arrows) on the targeted (asterisks; green in A’,B’) and untargeted sides of the dorsal neural tube in representative ContMO-electroporated embryos (A,B) and transverse sections (A”,B”). At 9s, Tspan18 expression is retained (arrowhead) on the FoxD3MO-targeted side of the neural tube (asterisk; C’, green) in embryos (C) and transverse sections (C”). At 10s, Tspan18 mRNA expression is downregulated (arrow), even on the FoxD3MO-targeted side (asterisk; F’, green) of the neural tube in embryos (D) and transverse sections (D”). (E) Bar graph representing the frequency of 8–9s embryos exhibiting sustained Tspan18 expression. (F) Quantification of Tspan18 mRNA levels following MO-mediated depletion of Snail2. Cad6B levels were analyzed as a positive control (Taneyhill et al., 2007). Results are reported as fold difference relative to that obtained with ContMO normalized to 1. Means and standard errors of fold differences were generated from 3 independent experimental cDNA replicates. The asterisk (*) located above the error bar denotes a significant difference in the fold change between Snail2MO and ContMO levels (p< 0.05). Black bars, Snail2 MO; white bars, ContMO.