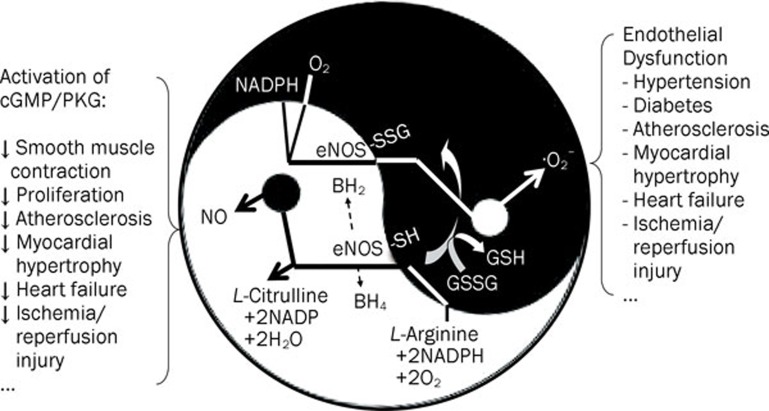
Figure 1.
Schematic representation of S-glutathionylation of eNOS as a molecular switch of eNOS function from generation of NO to production of superoxide anion. The endothelial NO synthase (eNOS) uses L-arginine to generate NO. NO activates the guanylate cyclase/cyclic GMP (cGMP)/protein kinase G (PKG) pathway and modulates protein properties and function through nitrosylation of tyrosine and thiol-groups of cysteine in proteins, which are effective protection mechanisms against oxidative stress. Under pathological conditions NO production and bioavailability could be attenuated due to decreased supply of L-arginine or oxidation of the cofactor tetrahydrobiopterin (BH4) of eNOS, leading to eNOS uncoupling and superoxide anion (·O2−) formation. Under oxidative stress, eNOS is S-glutathionylated through reversible thiol-disulfide exchange with oxidized glutathione (GSSG) or reaction of oxidant-induced protein thiolyl radicals with reduced glutathione (GSH). S-glutathionylation of eNOS in endothelial cells switch off NO synthesis and turns on ·O2− generation, which is a pivotal mechanism for many diseases associated with epithelial dysfunction.