FIGURE 3.
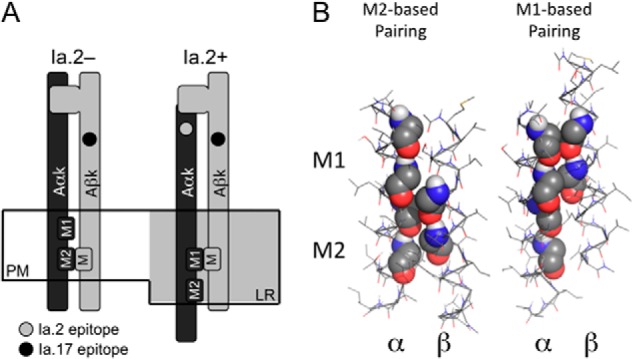
Differential transmembrane domain GXXXG motif pairing and Ia. 2+ I-Ak class II molecules. A, model of proposed GXXXG motif pairing of Ia.2+ and Ia.2− I-Ak class II molecules at the plasma membrane (PM). In the non-raft-resident Ia.2− molecules, the β chain GXXXG motif pairs with the α chain C-terminal M2 motif (M2 conformer). In the lipid raft (LR)-resident Ia.2+ molecules, the β chain GXXXG motif pairs with the α chain N-terminal M1 motif, supporting formation of a Ia.2+ class II conformation (M1 conformer). B, interactions between the TM domains of the wild type murine I-Ak MHC class II molecule (Table 1) were modeled in CHI, and low energy solutions were obtained in which the β GXXXG chain motif paired with either the α chain M1 motif or the α chain M2 motif (motif residues are shown in sphere representation).
