FIGURE 6.
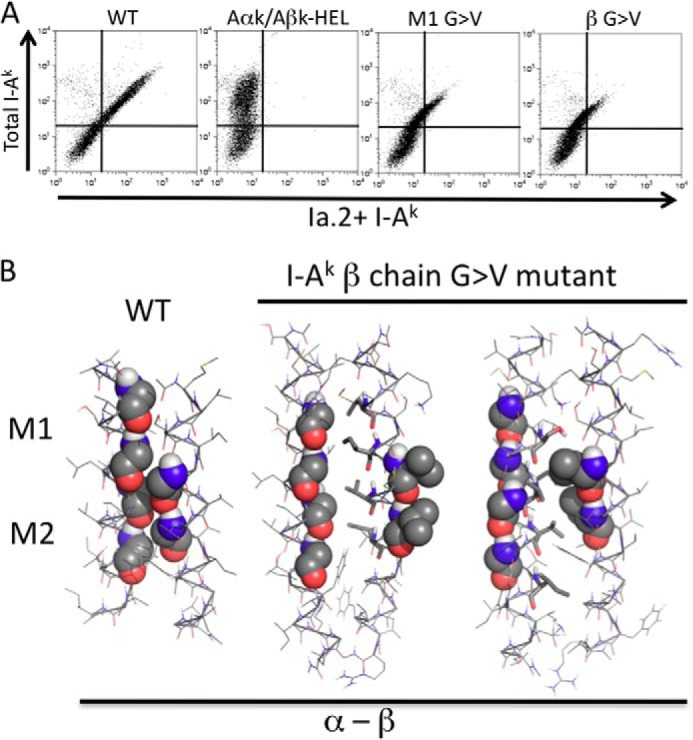
Decreased Ia. 2+ class II expression by mutation of I-Ak β chain GXXXG motif. A, CIITA-expressing 293T cells were transfected with the indicated I-Ak α (Aαk) and β (Aβk) chain cDNAs and Ia.2 versus Ia.17 expression determined as in Fig. 4. Shown are representative results from 1 of greater than 3 independent experiments. B, interactions between the TM domains of the murine I-Ak MHC class II β chain G → V mutant and wild type α chain were modeled in CHI. No low energy solutions in which the mutant β chain motif was paired with either the α chain M1 or the α chain M2 motifs were obtained. Instead, mutation of the β chain GXXXG led to exclusion of the motif from the heterodimer interface (center panel) in favor of use of an alternate face composed of hydrophobic residues (stick representation). Modeling runs also produced heterodimers in which the mutated β motif packs against an alternate face of the α chain composed of Ser, Val, and Ile residues (right panel). The M2-based pairing observed with WT class II is shown for reference.
