FIGURE 7.
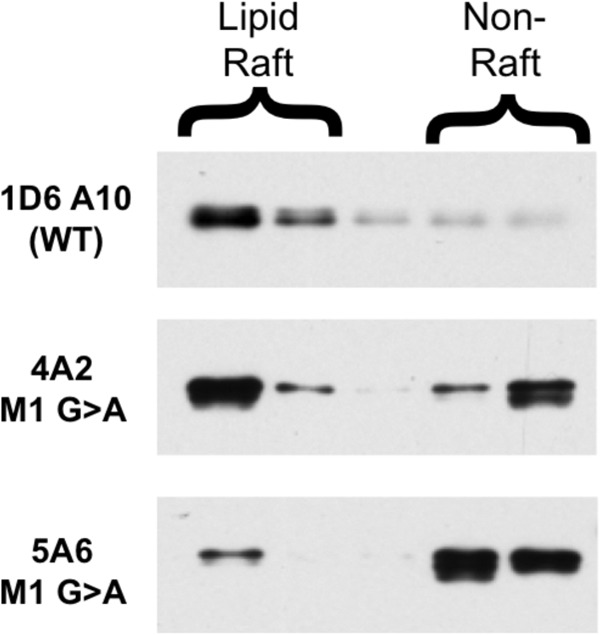
Decreased lipid raft partitioning of α chain M1 mutant I-Ak class II molecules. B cells expressing either WT or Aαk M1 G → A mutant I-Ak MHC class II molecules were solubilized in ice-cold 0.1% Triton X-100 to liberate lipid rafts (4A2 = Clone 1 from Fig. 4 and 5A6 = Clone 2). Lipid rafts were separated from non-raft material by sucrose density gradient centrifugation. I-Ak class II molecules were immunoprecipitated from each gradient fraction with the 11-5.2 mAb (which recognizes an epitope on the transfected Aαk chain, which is missing from the endogenous WT Aαd chain) and protein G-Sepharose. Immunoprecipitates were analyzed for class II molecules by Western blot with an anti-β chain antibody. Shown are representative results from 1 of 4 independent experiments (two in which class II was immunoprecipitated with 11-5.2 and detected by an anti-α chain Western blot (shown) and two in which 11-5.2-btn was bound to cell surface class II and detected by streptavidin Western blot (not shown)).
