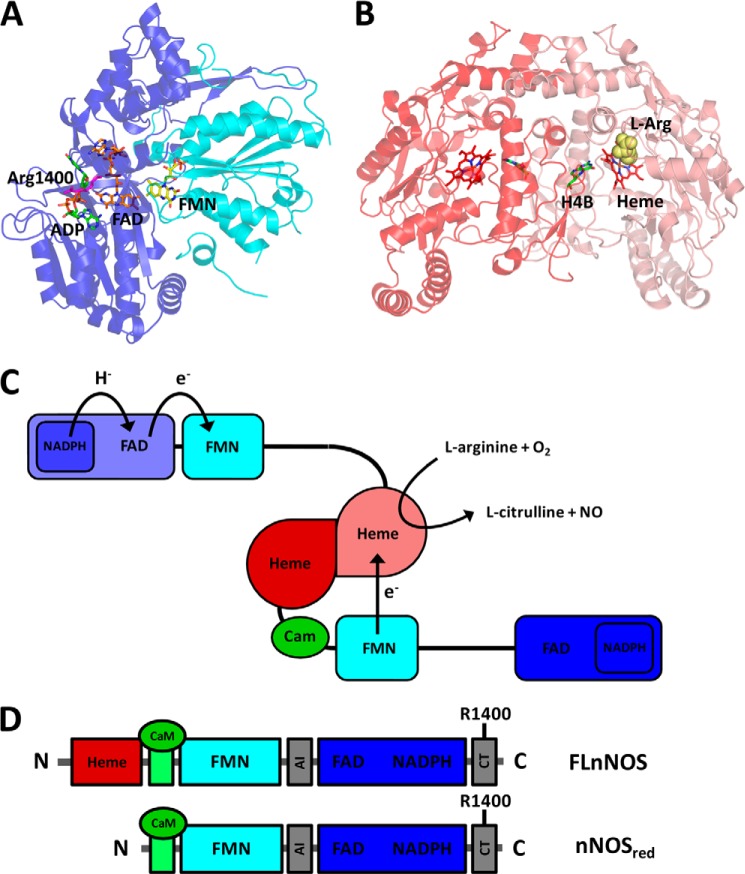
FIGURE 1.
Structural organization of nNOS. A, crystal structure of the nNOS diflavin reductase domain with bound ADP (PDB 1TLL) (10). The FAD-binding subdomain is highlighted in blue, and the FMN-binding subdomain is highlighted in cyan. The distance between the FAD and FMN cofactors is 15.5 Å. B, crystal structure of the nNOS oxygenase dimer with bound l-arginine (PDB 1ZVL) (52). The oxygenase and reductase domains are connected via a CaM-binding region. C, schematic representation of a NOS homodimer in the non-productive state without CaM bound, and its productive state with CaM bound. D, recombinant nNOS constructs used in this study. The CaM-binding domain is highlighted in green, and the two regions that harbor the autoinhibitory insert (AI) and the C-terminal tail (CT) regulatory sequences are highlighted in gray. FLnNOS is the full-length nNOS protein, including the N-terminal oxygenase domain, CaM-binding region, and the C-terminal diflavin reductase domain. nNOSred comprises the CaM-binding region and the C-terminal diflavin reductase domain (residues 695–1429). H4B, tetrahydrobiopterin.