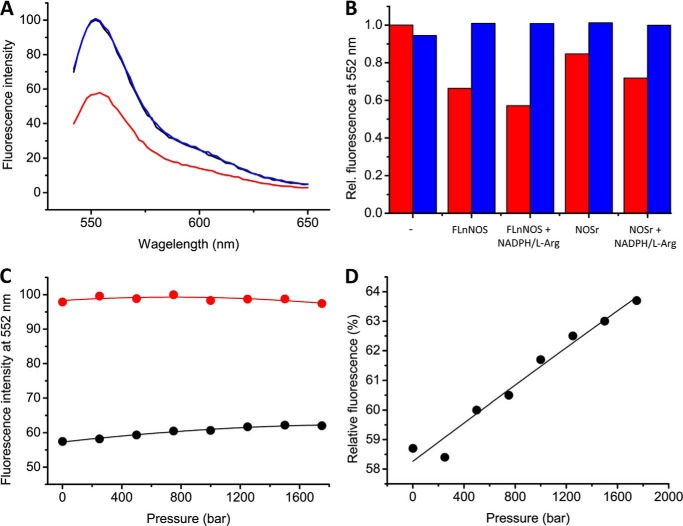
FIGURE 5.
Calmodulin binding to nNOS as a function of hydrostatic pressure. All experiments were carried out at room temperature in buffer (pH 7.6) supplemented with 0.3 mm CaCl2, 2 mm l-arginine, and 100 μm NADPH, Ca2+ was removed by the addition of 0.5 mm EDTA, as described under “Materials and Methods.” A, fluorescence emission spectra (λexc = 536 nm) of CaM-T34C-A532 (black line), CaM-T34C-A532 plus full-length nNOS in the presence of Ca2+ (red line), and CaM-T34C-A532 plus full-length nNOS after the addition of EDTA (blue line). B, fluorescence quenching of CaM-T34C-A532 at 552 nm by full-length nNOS and nNOSred in the resting state, or under turnover conditions (+NADPH/l-Arg), in the presence (red) or absence of Ca2+ (blue). C, pressure-dependent changes in fluorescence intensity of CaM-T34C-A532 upon binding of nNOS under turnover conditions, in the presence of Ca2+ (black) or EDTA (red). All data points were corrected for pressure-dependent changes in fluorescence in the absence of nNOS and fitted to Equation 2. D, relative fluorescence intensity of CaM-T34C-A532 in the presence of full-length nNOS and Ca2+ versus the same sample in the absence of Ca2+, as a function of hydrostatic pressure.