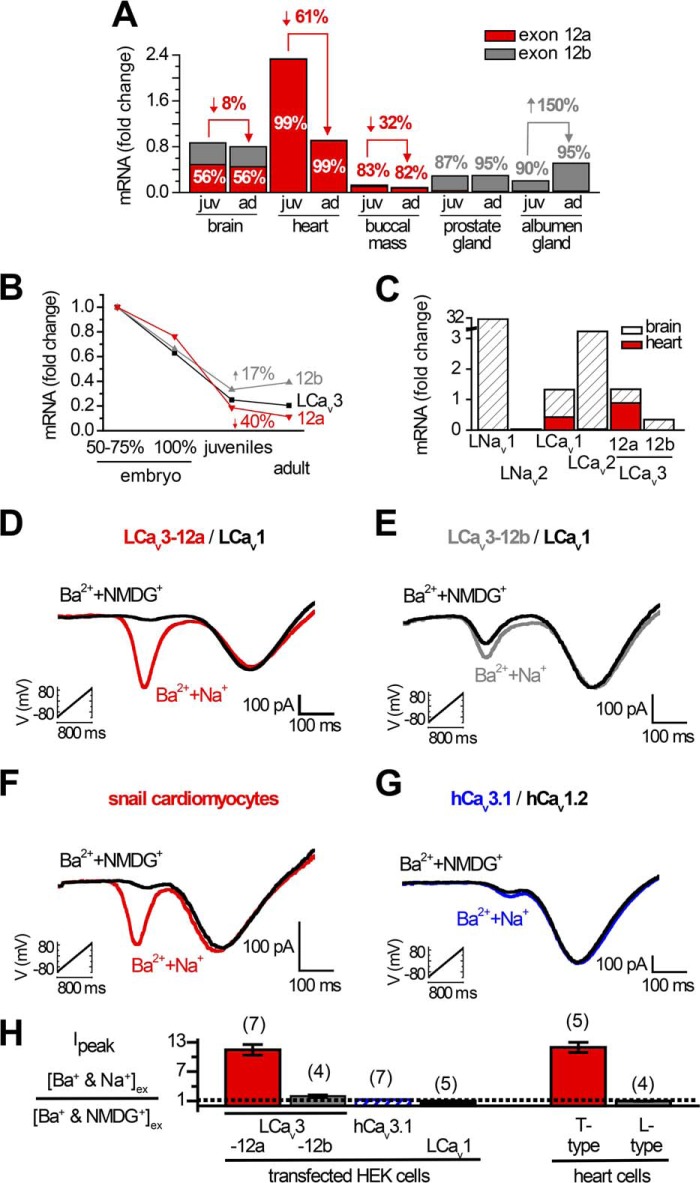
FIGURE 6.
Snails express two cardiac cation currents, the highly sodium-permeant T-type channel LCav3 and the calcium-selective L-type channel LCav1. A–C, quantitative RT-PCR standardized to Lymnaea HPRT1 control gene. A, relative mRNA expression levels of exon 12a and 12b in juvenile (juv) and adult (ad) snails, reflecting the almost exclusive expression of exon 12a in the snail heart and exon 12b in prostate and albumen glands. B, dramatic decline in LCav3 channel expression in whole animals can mostly be attributed to the decline of LCav3-12a expression in the developing heart. C, snail hearts lack mRNA expression of any sodium channel gene (LNav1 and LNav2). Snail hearts also lack any expression of the LCav2 synaptic non-L-type channel or the Cav3 T-type channel gene with exon 12b. Note the break in the y axis scale, illustrating that the range of expression of the sodium channel gene of LNav1 in the snail brain is ∼30-fold higher than the control HPRT1 gene, compared with the low levels of expression of the other calcium channel genes (which vary up to ∼3-fold higher than the control HPRT1 gene). D–G, ramp protocols (−110 to +100 mV in 1 s) carried out in the presence of external solutions as follows: 2 mm Ba2+ and 100 mm Na+ ions or 2 mm Ba2+ and 100 mm NMDG+ reveal a covert sodium-permeant, T-type current superimposed on the Ba2+ current in cardiomyocytes in the presence of a sodium-impermeant, L-type current (F). The sodium-permeant T-type currents in cardiomyocytes are indistinguishable from mammalian HEK-293T cells transfected with LCav3 with exon 12a with co-expression of LCav1 and accessory subunits (α2δ and β2a) (D). E, LCav3 with exon 12b; G, human Cav3.1 has some Na+ permeability but is not as Na+-permeant as the LCav3-12a variant expressed in cardiomyocytes. H, bar graph ± S.E., including sample data in D–G.