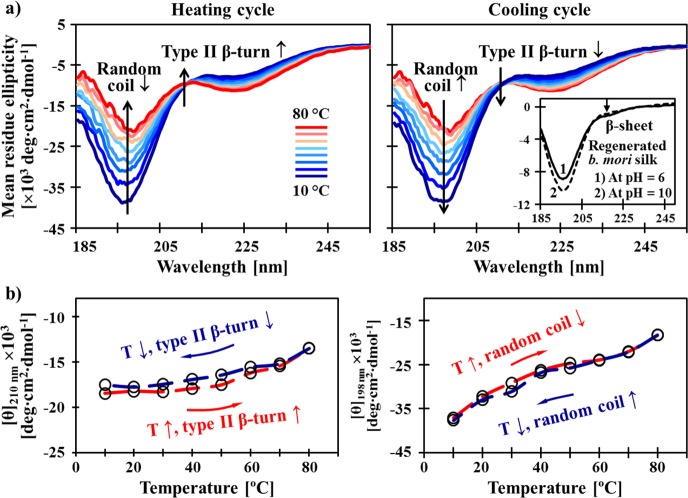
Figure 3.
Molecular origin of the reversible thermal response of the recombinant S4E8G protein polymers. A heating-induced conformational transition in the elastin-like domains of S4E8G-18-mer, from random coil to type II β-turn, was revealed by variable-temperature CD spectroscopy. (a) S4E8G-18-mers (0.3 mg/mL in H2O) at low temperatures were found to adopt random coil-dominated structures, containing small amounts of β-conformations. The inset shows reference CD spectra of regenerated B. mori silk (0.05 wt % in H2O), adopting 100% random coil at pH 10 and containing about 5% of β-sheets41 at pH 6. The evolution of a positive ellipticity band with a maximum at 210 nm during heating confirms the growth of type II β-turn.44 Note that an isodichroic point occurred at ∼213 nm, implying two-state solution behavior,45 and that the β-structures in the silk-like domains were not affected by temperature. (b) Thermally induced conversion between random coil and β-turn in the elastin-like domains was demonstrated to be rapidly reversible in the hysteresis loops.