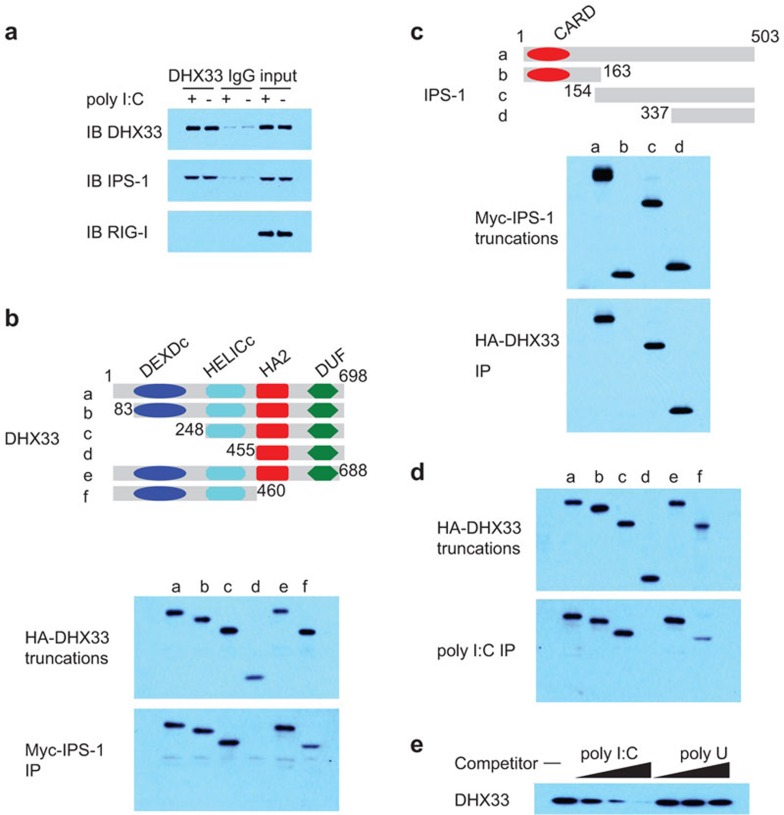
Figure 5.
DHX33 interacts with both poly I:C and IPS-1. (a) IB of the indicated proteins precipitated with an anti-DHX33 antibody from whole-cell lysates of D2SC cells that were unstimulated (−) or stimulated with long poly I:C (+). An IgG antibody served as a control. (b) Top: schematic representation of DHX33 and its serial truncations. DExDc: Asp–Glu–Ala–Asp box motif; Helic C: helicase C-terminal domain. HA2: helicase-associated domain. DUF: domain of unknown function. Numbers denote amino-acid residues. Middle panel: immunoblot of purified HA-DHX33 truncated proteins using an anti-HA antibody (a–f). Bottom: immunoblot using an anti-HA antibody of proteins precipitated (IP) with an anti-Myc antibody from a mixture of an HA-DHX33 protein (a–f) individually incubated with a Myc-IPS-1 fusion protein. (c) Top: schematic representation of IPS-1 and its serial truncations. CARD: the caspase activation and recruitment domain. Numbers denote amino-acid residues. Middle panel: immunoblot using an anti-Myc antibody of purified Myc-IPS-1 truncated proteins (a–d). Bottom: immunoblot using an anti-Myc antibody of proteins precipitated (IP) from a mixture of a Myc-IPS-1 protein (a–d) individually incubated with an HA-DHX33 fusion protein. (d) Immunoblot using an anti-HA antibody of pulldown assays in which purified, serial truncations of HA-DHX33 (a–f) were individually incubated with biotinylated poly I:C, followed by the addition of NeutrAvidin beads. (e) Immunoblot using an anti-HA antibody of pulldown competition assay products in which 0.5, 5 or 50 µg/ml poly I:C or poly U was added to a mixture of HA-DHX33 plus biotinylated poly I:C, followed by the addition of NeutrAvidin beads. IB, immunoblot; IP, immunoprecipitation.